当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Oxidation of CO on Cu Single Crystals under Alkaline Conditions
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-10-15 , DOI: 10.1021/acsenergylett.0c01751 Aarti Tiwari 1 , Hendrik H. Heenen 1 , Anton Simon Bjørnlund 1 , Degenhart Hochfilzer 1 , Karen Chan 1 , Sebastian Horch 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-10-15 , DOI: 10.1021/acsenergylett.0c01751 Aarti Tiwari 1 , Hendrik H. Heenen 1 , Anton Simon Bjørnlund 1 , Degenhart Hochfilzer 1 , Karen Chan 1 , Sebastian Horch 1, 2
Affiliation
|
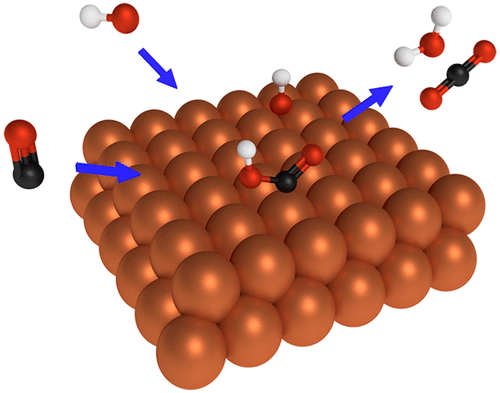
We perform a joint experimental–theoretical study of the electrochemical oxidation of CO on copper (Cu) under alkaline conditions. Using cyclic voltammetry on Cu single-crystal surfaces, we demonstrate that both Cu terraces and steps show CO oxidation activity at potentials just slightly positive (0.03–0.14 V) of the thermodynamic equilibrium potential. The overpotentials are 0.23–0.12 V lower than that of gold (≈0.26 V), which until now has been considered to be the most active catalyst for this process. Our theoretical calculations suggest that Cu’s activity arises from the advantageous combination of simultaneous *CO and *OH adsorption under CO oxidation potentials and surmountable *CO–*OH coupling barriers. Experimentally observed onset potentials are in agreement with the computed onsets of *OH adsorption. We furthermore show that the onsets of *OH adsorption on steps are more affected by *CO–*OH interactions than on terraces due to a stronger competitive adsorption. Overall, Cu(100) shows the lowest overpotential (0.03 V) of the facets considered.
中文翻译:

碱性条件下CO在Cu单晶上的电化学氧化
我们进行了碱性条件下CO在铜(Cu)上的电化学氧化的联合实验理论研究。在铜单晶表面上使用循环伏安法,我们证明了铜阶跃和阶梯都显示出在热力学平衡电势略微为正(0.03-0.14 V)的电势下的CO氧化活性。过电势比金(≈0.26V)低0.23–0.12 V,迄今为止,金被认为是该过程中活性最高的催化剂。我们的理论计算表明,铜的活性来自同时* CO和* OH在CO氧化电位下的吸附和可克服的* CO- * OH耦合势垒的有利结合。实验观察到的起始电位与* OH吸附的计算起始相符。我们进一步表明,由于强竞争吸附,台阶上* OH吸附的开始受* CO- * OH相互作用的影响要比台阶上更多。总体而言,Cu(100)在所考虑的方面显示出最低的过电势(0.03 V)。
更新日期:2020-11-13
中文翻译:

碱性条件下CO在Cu单晶上的电化学氧化
我们进行了碱性条件下CO在铜(Cu)上的电化学氧化的联合实验理论研究。在铜单晶表面上使用循环伏安法,我们证明了铜阶跃和阶梯都显示出在热力学平衡电势略微为正(0.03-0.14 V)的电势下的CO氧化活性。过电势比金(≈0.26V)低0.23–0.12 V,迄今为止,金被认为是该过程中活性最高的催化剂。我们的理论计算表明,铜的活性来自同时* CO和* OH在CO氧化电位下的吸附和可克服的* CO- * OH耦合势垒的有利结合。实验观察到的起始电位与* OH吸附的计算起始相符。我们进一步表明,由于强竞争吸附,台阶上* OH吸附的开始受* CO- * OH相互作用的影响要比台阶上更多。总体而言,Cu(100)在所考虑的方面显示出最低的过电势(0.03 V)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号