当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
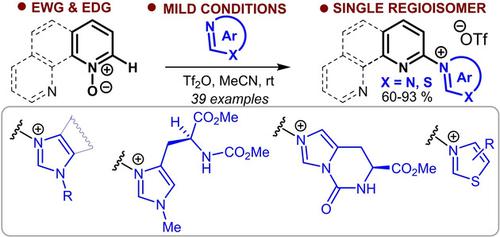
Reaction of Pyridine‐N‐Oxides with Tertiary sp2‐N‐Nucleophiles: An Efficient Synthesis of Precursors for N‐(Pyrid‐2‐yl)‐Substituted N‐Heterocyclic Carbenes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-10-14 , DOI: 10.1002/adsc.202001063 Dmitry I. Bugaenko 1 , Marina A. Yurovskaya 1 , Alexander V. Karchava 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-10-14 , DOI: 10.1002/adsc.202001063 Dmitry I. Bugaenko 1 , Marina A. Yurovskaya 1 , Alexander V. Karchava 1
Affiliation
|
N‐(Pyrid‐2‐yl)‐substituted azolium and pyridinium salts, precursors for hybrid NHC‐containing ligands, were obtained with excellent regioselectivity, employing a deoxygenative CH‐functionalization of pyridine‐N‐oxides with substituted imidazoles, thiazoles, and pyridine. Unlike the traditional SNAr‐based methods, this approach provides high yields for substrates bearing substituents of different electronic nature. The utility of azolium and pyridinium salts thus prepared was also highlighted by the synthesis of pyridyl‐substituted imidazolyl‐2‐thione, benzodiazepine as well as 2‐aminopyridines.
中文翻译:

吡啶-N-氧化物与sp2-N-亲核叔胺的反应:N-(吡啶-2-基)取代的N-杂环卡宾的前体的有效合成
通过使用取代的咪唑,噻唑和吡啶对吡啶-N-氧化物进行脱氧CH-官能团化反应,获得了具有出色的区域选择性的N-(吡啶-2-基)-取代的azo盐和吡啶鎓盐,具有优异的区域选择性。。与传统的基于S N Ar的方法不同,此方法可为带有不同电子性质取代基的底物提供高收率。吡啶基取代的咪唑基-2-硫酮,苯并二氮杂以及2-氨基吡啶的合成也突出了如此制备的偶氮盐和吡啶鎓盐的用途。
更新日期:2020-12-22
中文翻译:

吡啶-N-氧化物与sp2-N-亲核叔胺的反应:N-(吡啶-2-基)取代的N-杂环卡宾的前体的有效合成
通过使用取代的咪唑,噻唑和吡啶对吡啶-N-氧化物进行脱氧CH-官能团化反应,获得了具有出色的区域选择性的N-(吡啶-2-基)-取代的azo盐和吡啶鎓盐,具有优异的区域选择性。。与传统的基于S N Ar的方法不同,此方法可为带有不同电子性质取代基的底物提供高收率。吡啶基取代的咪唑基-2-硫酮,苯并二氮杂以及2-氨基吡啶的合成也突出了如此制备的偶氮盐和吡啶鎓盐的用途。



























 京公网安备 11010802027423号
京公网安备 11010802027423号