Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The molecular species responsible for α1‐antitrypsin deficiency are suppressed by a small molecule chaperone
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-15 , DOI: 10.1111/febs.15597 Riccardo Ronzoni 1 , Nina Heyer-Chauhan 1 , Annamaria Fra 2 , Andrew C Pearce 3 , Martin Rüdiger 3 , Elena Miranda 4 , James A Irving 1 , David A Lomas 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-15 , DOI: 10.1111/febs.15597 Riccardo Ronzoni 1 , Nina Heyer-Chauhan 1 , Annamaria Fra 2 , Andrew C Pearce 3 , Martin Rüdiger 3 , Elena Miranda 4 , James A Irving 1 , David A Lomas 1
Affiliation
|
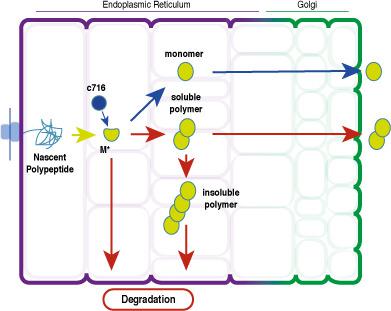
The formation of ordered Z (Glu342Lys) α1‐antitrypsin polymers in hepatocytes is central to liver disease in α1‐antitrypsin deficiency. In vitro experiments have identified an intermediate conformational state (M*) that precedes polymer formation, but this has yet to be identified in vivo. Moreover, the mechanism of polymer formation and their fate in cells have been incompletely characterised. We have used cell models of disease in conjunction with conformation‐selective monoclonal antibodies and a small molecule inhibitor of polymerisation to define the dynamics of polymer formation, accumulation and secretion. Pulse‐chase experiments demonstrate that Z α1‐antitrypsin accumulates as short‐chain polymers that partition with soluble cellular components and are partially secreted by cells. These precede the formation of larger, insoluble polymers with a longer half‐life (10.9 ± 1.7 h and 20.9 ± 7.4 h for soluble and insoluble polymers, respectively). The M* intermediate (or a by‐product thereof) was identified in the cells by a conformation‐specific monoclonal antibody. This was completely abrogated by treatment with the small molecule, which also blocked the formation of intracellular polymers. These data allow us to conclude that the M* conformation is central to polymerisation of Z α1‐antitrypsin in vivo; preventing its accumulation represents a tractable approach for pharmacological treatment of this condition; polymers are partially secreted; and polymers exist as two distinct populations in cells whose different dynamics have likely consequences for the aetiology of the disease.
中文翻译:

导致 α1-抗胰蛋白酶缺乏的分子种类被小分子伴侣抑制
肝细胞中有序 Z (Glu342Lys) α 1抗胰蛋白酶聚合物的形成是 α 1 抗胰蛋白酶缺乏症引起的肝脏疾病的核心。体外实验已经确定了聚合物形成之前的中间构象状态(M*),但这尚未在体内确定。此外,聚合物形成的机制及其在细胞中的命运尚未完全表征。我们将疾病细胞模型与构象选择性单克隆抗体和小分子聚合抑制剂结合使用,以定义聚合物形成、积累和分泌的动力学。脉冲追踪实验表明,Z α 1 -抗胰蛋白酶以短链聚合物的形式积累,与可溶性细胞成分分开,并由细胞部分分泌。这些先于形成较大的不溶性聚合物,具有较长的半衰期(可溶性和不溶性聚合物分别为 10.9 ± 1.7 小时和 20.9 ± 7.4 小时)。通过构象特异性单克隆抗体在细胞中鉴定出 M* 中间体(或其副产物)。通过小分子处理完全消除了这种现象,同时也阻止了细胞内聚合物的形成。这些数据使我们得出结论,M* 构象是 Z α 1抗胰蛋白酶体内聚合的核心;防止其积累代表了一种药物治疗这种疾病的易处理方法;聚合物被部分分泌;聚合物在细胞中以两个不同的群体存在,其不同的动力学可能对疾病的病因产生影响。
更新日期:2020-10-15
中文翻译:

导致 α1-抗胰蛋白酶缺乏的分子种类被小分子伴侣抑制
肝细胞中有序 Z (Glu342Lys) α 1抗胰蛋白酶聚合物的形成是 α 1 抗胰蛋白酶缺乏症引起的肝脏疾病的核心。体外实验已经确定了聚合物形成之前的中间构象状态(M*),但这尚未在体内确定。此外,聚合物形成的机制及其在细胞中的命运尚未完全表征。我们将疾病细胞模型与构象选择性单克隆抗体和小分子聚合抑制剂结合使用,以定义聚合物形成、积累和分泌的动力学。脉冲追踪实验表明,Z α 1 -抗胰蛋白酶以短链聚合物的形式积累,与可溶性细胞成分分开,并由细胞部分分泌。这些先于形成较大的不溶性聚合物,具有较长的半衰期(可溶性和不溶性聚合物分别为 10.9 ± 1.7 小时和 20.9 ± 7.4 小时)。通过构象特异性单克隆抗体在细胞中鉴定出 M* 中间体(或其副产物)。通过小分子处理完全消除了这种现象,同时也阻止了细胞内聚合物的形成。这些数据使我们得出结论,M* 构象是 Z α 1抗胰蛋白酶体内聚合的核心;防止其积累代表了一种药物治疗这种疾病的易处理方法;聚合物被部分分泌;聚合物在细胞中以两个不同的群体存在,其不同的动力学可能对疾病的病因产生影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号