当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic study of ethanol impact on gemcitabine binding to cucurbit[7]uril in aqueous solutions
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jct.2020.106317 Adam Buczkowski , Paweł Tokarz , Bartłomiej Palecz
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jct.2020.106317 Adam Buczkowski , Paweł Tokarz , Bartłomiej Palecz
|
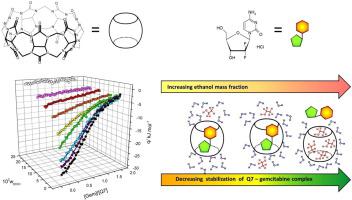
Abstract The process of complexation of gemcitabine cation by cucurbituril Q7 was studied in an aqueous formate buffer (pH= p K a = 3.8) with increasing ethanol mass fraction wEtOH using ITC calorimetric titration technique. The equilibrium constant K and thermodynamic functions ( Δ H , Δ S , Δ G ) describing the process of gemcitabine binding by cucurbituril Q7 were calculated. The determined parameters indicate that in the range of ethanol mass fraction physiologically tolerated by the human body (wEtOH≤0.005), the interaction energetics of cucurbituril Q7 with gemcitabine are similar to those observed in a buffer environment without the addition of alcohol. This suggests that the ethanol present in the patient's body is not a significant contraindication to the use of cucurbituril Q7 as a supramolecular gemcitabine nano-container for biomedical applications. However, higher ethanol mass fraction in solution clearly weaken drug interaction with cucurbituril Q7. Therefore, for potential technological applications using cucurbituril Q7 as a gemcitabine carrier, the ethanol mass fraction wEtOH should be lower than 0.1.
中文翻译:

乙醇对水溶液中吉西他滨结合葫芦[7]脲的热力学研究
摘要 使用 ITC 量热滴定技术,随着乙醇质量分数 wEtOH 在含水甲酸盐缓冲液 (pH= p K a = 3.8) 中,葫芦脲 Q7 络合吉西他滨阳离子的过程进行了研究。计算了描述葫芦脲 Q7 与吉西他滨结合过程的平衡常数 K 和热力学函数(ΔH、ΔS、ΔG)。确定的参数表明,在人体生理耐受的乙醇质量分数范围内(wEtOH≤0.005),葫芦脲Q7与吉西他滨的相互作用能量学与在不添加酒精的缓冲环境中观察到的相似。这表明患者体内的乙醇 对于使用葫芦脲 Q7 作为生物医学应用的超分子吉西他滨纳米容器,身体并不是一个明显的禁忌症。然而,溶液中较高的乙醇质量分数明显削弱了与葫芦脲 Q7 的药物相互作用。因此,对于使用葫芦脲 Q7 作为吉西他滨载体的潜在技术应用,乙醇质量分数 wEtOH 应低于 0.1。
更新日期:2021-02-01
中文翻译:

乙醇对水溶液中吉西他滨结合葫芦[7]脲的热力学研究
摘要 使用 ITC 量热滴定技术,随着乙醇质量分数 wEtOH 在含水甲酸盐缓冲液 (pH= p K a = 3.8) 中,葫芦脲 Q7 络合吉西他滨阳离子的过程进行了研究。计算了描述葫芦脲 Q7 与吉西他滨结合过程的平衡常数 K 和热力学函数(ΔH、ΔS、ΔG)。确定的参数表明,在人体生理耐受的乙醇质量分数范围内(wEtOH≤0.005),葫芦脲Q7与吉西他滨的相互作用能量学与在不添加酒精的缓冲环境中观察到的相似。这表明患者体内的乙醇 对于使用葫芦脲 Q7 作为生物医学应用的超分子吉西他滨纳米容器,身体并不是一个明显的禁忌症。然而,溶液中较高的乙醇质量分数明显削弱了与葫芦脲 Q7 的药物相互作用。因此,对于使用葫芦脲 Q7 作为吉西他滨载体的潜在技术应用,乙醇质量分数 wEtOH 应低于 0.1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号