当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic investigation of G2 and G4 siloxane dendrimers with trimethylsilyl terminal groups
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jct.2020.106318 Semen S. Sologubov , Alexey V. Markin , Yuliya A. Sarmini , Natalia N. Smirnova , Konstantin L. Boldyrev , Elena A. Tatarinova , Ivan B. Meshkov , Aziz M. Muzafarov
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jct.2020.106318 Semen S. Sologubov , Alexey V. Markin , Yuliya A. Sarmini , Natalia N. Smirnova , Konstantin L. Boldyrev , Elena A. Tatarinova , Ivan B. Meshkov , Aziz M. Muzafarov
|
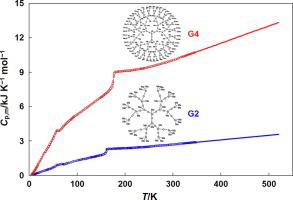
Abstract In this work, we report results of the calorimetric study of the second (G2[OSi(CH3)3]12) and fourth (G4[OSi(CH3)3]48) generation siloxane dendrimers with trimethylsilyl terminal groups. The heat capacities of dendrimers were precisely measured in the temperature range T = (5–520) K using a fully automated adiabatic calorimeter and a heat-flux differential scanning calorimeter. In the above temperature interval, the physical transformations of the studied compounds were detected, and its thermodynamic characteristics were determined. The fundamental thermodynamic functions (the enthalpy [H°(T) − H°(0)], the entropy [S°(T) − S°(0)], the Gibbs energy [G°(T) − H°(0)]) of dendrimers were calculated over the range from T → 0 to 520 K using the experimentally determined heat capacities of the investigated compounds. The standard entropies of formation of dendrimers G2[OSi(CH3)3]12 and G4[OSi(CH3)3]48 were evaluated at T = 298.15 K. The obtained thermodynamic data of the investigated dendrimers were compared with those of the studied earlier siloxane dendrimers G1[OSi(CH3)3]6 and G3[OSi(CH3)3]24, which represent the structurally related homologous series of organosilicon dendrimers. As a result, the dependences between thermodynamic properties of the studied siloxane dendrimers and their molecular mass were established.
中文翻译:

具有三甲基甲硅烷基端基的 G2 和 G4 硅氧烷树枝状聚合物的热力学研究
摘要 在这项工作中,我们报告了具有三甲基甲硅烷基端基的第二代 (G2[OSi(CH3)3]12) 和第四代 (G4[OSi(CH3)3]48) 硅氧烷树枝状聚合物的量热研究结果。使用全自动绝热量热仪和热通量差示扫描量热仪在 T = (5-520) K 温度范围内精确测量树枝状聚合物的热容量。在上述温度区间内,检测了所研究化合物的物理转变,并确定了其热力学特性。基本热力学函数(焓 [H°(T) − H°(0)]、熵 [S°(T) − S°(0)]、吉布斯能量 [G°(T) − H°( 0)]) 的树枝状聚合物在 T → 0 到 520 K 的范围内使用所研究化合物的实验确定的热容进行计算。在 T = 298.15 K 下评估了树枝状聚合物 G2[OSi(CH3)3]12 和 G4[OSi(CH3)3]48 形成的标准熵。 将所研究树枝状聚合物的热力学数据与之前研究的数据进行比较硅氧烷树枝状聚合物 G1[OSi(CH3)3]6 和 G3[OSi(CH3)3]24,代表结构相关的有机硅树枝状聚合物的同源系列。结果,建立了所研究的硅氧烷树枝状聚合物的热力学性质与其分子量之间的依赖性。
更新日期:2021-02-01
中文翻译:

具有三甲基甲硅烷基端基的 G2 和 G4 硅氧烷树枝状聚合物的热力学研究
摘要 在这项工作中,我们报告了具有三甲基甲硅烷基端基的第二代 (G2[OSi(CH3)3]12) 和第四代 (G4[OSi(CH3)3]48) 硅氧烷树枝状聚合物的量热研究结果。使用全自动绝热量热仪和热通量差示扫描量热仪在 T = (5-520) K 温度范围内精确测量树枝状聚合物的热容量。在上述温度区间内,检测了所研究化合物的物理转变,并确定了其热力学特性。基本热力学函数(焓 [H°(T) − H°(0)]、熵 [S°(T) − S°(0)]、吉布斯能量 [G°(T) − H°( 0)]) 的树枝状聚合物在 T → 0 到 520 K 的范围内使用所研究化合物的实验确定的热容进行计算。在 T = 298.15 K 下评估了树枝状聚合物 G2[OSi(CH3)3]12 和 G4[OSi(CH3)3]48 形成的标准熵。 将所研究树枝状聚合物的热力学数据与之前研究的数据进行比较硅氧烷树枝状聚合物 G1[OSi(CH3)3]6 和 G3[OSi(CH3)3]24,代表结构相关的有机硅树枝状聚合物的同源系列。结果,建立了所研究的硅氧烷树枝状聚合物的热力学性质与其分子量之间的依赖性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号