Talanta ( IF 5.6 ) Pub Date : 2020-10-15 , DOI: 10.1016/j.talanta.2020.121776 Fenglong Jiao , Fangyuan Gao , Yuanyuan Liu , Zhiya Fan , Xiaochao Xiang , Chaoshuang Xia , Yayao Lv , Yuping Xie , Haihong Bai , Wanjun Zhang , Weijie Qin , Xiaohong Qian
|
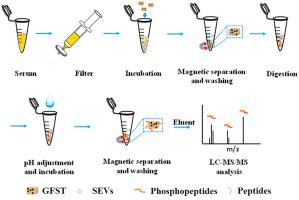
Small extracellular vesicles (SEVs), are cell-derived, membrane-enclosed nanometer-sized vesicles that play vital roles in many biological processes. Recent years, more and more evidences proved that small EVs have close relationship with many diseases such as cancers and Alzheimer's disease. The use of phosphoproteins in SEVs as potential biomarkers is a promising new choice for early diagnosis and prognosis of cancer. However, current techniques for SEVs isolation still facing many challenges, such as highly instrument dependent, time consuming and insufficient purity. Furthermore, complex enrichment procedures and low microgram amounts of proteins available from clinical sources largely limit the throughput and the coveage depth of SEVs phosphoproteome mapping. Here, we synthesized Ti4+-modified magnetic graphene-oxide composites (GFST) and developed a “one-material” strategy for facile and efficient phosphoproteome enrichment and identification in SEVs from human serum. By taking advantage of chelation and electrostatic interactions between metal ions and phosphate groups, GFST shows excellent performance in both SEVs isolation and phosphopeptide enrichment. Close to 85% recovery is achieved within a few minutes by simple incubation with GFST and magnetic separation. Proteome profiling of the isolated serum SEVs without phosphopeptide enrichment results in 515 proteins, which is approximately one-fold more than those otained by ultracentrifugation or coprecipitation kits. Further application of GFST in one-material-based enrichment led to identification of 859 phosphosites in 530 phosphoproteins. Kinase-substrate correlation analysis reveals enriched substrates of CAMK in serum SEVs phosphoproteome. Therefore, we expect that the low instrument dependency and the limited sample requirement of this new strategy may facilitate clinical investigations in SEV-based transportation of abnormal kinases and substrates for drug target discovery and cancer monitoring.
中文翻译:

串联富集小细胞外囊泡磷酸蛋白质组的简便“单一材料”策略
小型细胞外囊泡(SEVs)是细胞衍生的,膜包裹的纳米级囊泡,在许多生物过程中都起着至关重要的作用。近年来,越来越多的证据证明小型电动汽车与许多疾病如癌症和阿尔茨海默氏病有着密切的关系。在SEV中将磷蛋白用作潜在的生物标志物是癌症早期诊断和预后的有希望的新选择。但是,当前用于SEV隔离的技术仍然面临许多挑战,例如高度依赖仪器,耗时且纯度不足。此外,复杂的富集程序和可从临床来源获得的低毫克量的蛋白质在很大程度上限制了SEV磷酸化蛋白质组图谱的通量和覆盖深度。在这里,我们合成了Ti 4+改良的磁性氧化石墨烯复合材料(GFST),并开发了一种“单一材料”策略,可从人血清中简便有效地富集和鉴定SEV中的磷酸化蛋白质组。通过利用金属离子与磷酸基团之间的螯合和静电相互作用,GFST在SEV分离和磷酸肽富集方面均表现出出色的性能。通过与GFST的简单孵育和磁力分离,在几分钟之内即可达到近85%的回收率。没有磷酸肽富集的分离血清SEV的蛋白质组分析产生了515种蛋白质,比超速离心或共沉淀试剂盒获得的蛋白质约高一倍。GFST在基于单一材料的富集中的进一步应用导致鉴定了530个磷蛋白中的859个磷酸位。激酶-底物相关性分析揭示了血清SEVs磷酸化蛋白质组中CAMK的丰富底物。因此,我们期望这种新策略的低仪器依赖性和有限的样品需求可能有助于基于SEV的异常激酶和底物转运的临床研究,以用于药物靶标发现和癌症监测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号