Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-10-15 , DOI: 10.1016/j.jmb.2020.10.014 Ananda Ayyappan Jaguva Vasudevan , Kannan Balakrishnan , Christoph G.W. Gertzen , Fanni Borvető , Zeli Zhang , Anucha Sangwiman , Ulrike Held , Caroline Küstermann , Sharmistha Banerjee , Gerald G. Schumann , Dieter Häussinger , Ignacio G. Bravo , Holger Gohlke , Carsten Münk
|
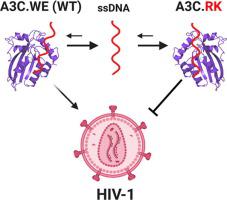
APOBEC3 deaminases (A3s) provide mammals with an anti-retroviral barrier by catalyzing dC-to-dU deamination on viral ssDNA. Within primates, A3s have undergone a complex evolution via gene duplications, fusions, arms race, and selection. Human APOBEC3C (hA3C) efficiently restricts the replication of viral infectivity factor (vif)-deficient Simian immunodeficiency virus (SIVΔvif), but for unknown reasons, it inhibits HIV-1Δvif only weakly. In catarrhines (Old World monkeys and apes), the A3C loop 1 displays the conserved amino acid pair WE, while the corresponding consensus sequence in A3F and A3D is the largely divergent pair RK, which is also the inferred ancestral sequence for the last common ancestor of A3C and of the C-terminal domains of A3D and A3F in primates. Here, we report that modifying the WE residues in hA3C loop 1 to RK leads to stronger interactions with substrate ssDNA, facilitating catalytic function, which results in a drastic increase in both deamination activity and in the ability to restrict HIV-1 and LINE-1 replication. Conversely, the modification hA3F_WE resulted only in a marginal decrease in HIV-1Δvif inhibition. We propose that the two series of ancestral gene duplications that generated A3C, A3D-CTD and A3F-CTD allowed neo/subfunctionalization: A3F-CTD maintained the ancestral RK residues in loop 1, while diversifying selection resulted in the RK → WE modification in Old World anthropoids’ A3C, possibly allowing for novel substrate specificity and function.
中文翻译:

APOBEC3C的回路1调节其对HIV-1的抗病毒活性
APOBEC3脱氨基酶(A3s)通过催化病毒ssDNA上的dC至dU脱氨作用为哺乳动物提供抗逆转录病毒屏障。在灵长类动物中,A3通过基因复制,融合,军备竞赛和选择经历了复杂的进化。人类APOBEC3C(hA3C)有效限制病毒感染因子(复制VIF)缺陷猴免疫缺陷病毒(SIVΔ VIF),但不知什么原因,它抑制HIV-1Δ VIF只有弱。在卡他汀类药物(旧世界的猴子和猿猴)中,A3C环1显示保守的氨基酸对WE,而A3F和A3D中相应的共有序列是很大程度上不同的RK,它也是最后一个祖先的推断祖先序列。在灵长类动物中的A3C和A3D和A3F的C端结构域。在这里,我们报告说,将hA3C环1中的WE残基修饰为RK会导致与底物ssDNA的相互作用更强,促进催化功能,从而导致脱氨活性以及限制HIV-1和LINE-1的能力急剧增加。复制。相反,修改hA3F_WE结果仅在HIV-1Δ稍减VIF抑制。我们建议产生A3C,A3D-CTD和A3F-CTD的两个祖先基因重复序列允许neo / subfunctionalization:A3F-CTD保留回路1中的祖先RK残基,而多样化的选择导致RK→WE在Old中进行修饰世界类人猿的A3C,可能允许新的底物特异性和功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号