Journal of Immunological Methods ( IF 1.6 ) Pub Date : 2020-10-15 , DOI: 10.1016/j.jim.2020.112901 Rachael E Whaley 1 , Sarah Ameny 1 , Tanvi Arkatkar 1 , Aaron Seese 1 , Abigail Wall 1 , Iram Khan 2 , Joseph J Carter 3 , Erin M Scherer 4 , David J Rawlings 5 , Denise A Galloway 3 , M Juliana McElrath 6 , Kristen W Cohen 1 , Andrew T McGuire 7
|
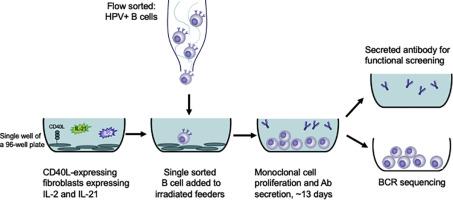
The isolation of human monoclonal antibodies (mAbs) arising from natural infection with human pathogens has proven to be a powerful technology, facilitating the understanding of the host response to infection at a molecular level. mAbs can reveal sites of vulnerability on pathogens and illuminate the biological function of the antigenic targets. Moreover, mAbs have the potential to be used directly for therapeutic applications such as passive delivery to prevent infection in susceptible target populations, and as treatment of established infection. The isolation of antigen-specific B cells from vaccine trials can also assist in deciphering whether the desired B cells are being targeted by a given vaccine. Several different processes have been developed to isolate mAbs, but all are generally labor-intensive and result in varying degrees of efficiency. Here, we describe the development of a cost-effective feeder cell line that stably expresses CD40-ligand, interleukin-2 and interleukin-21. Sorting of single B cells onto a layer of irradiated feeder cells sustained antibody production that permits functional screening of secreted antibodies in a manner that enables subsequent recovery of B cells for recombinant antibody cloning. As a proof of concept, we show that this approach can be used to isolate B cells that secrete antibodies that neutralize human papilloma virus (HPV) from participants of an HPV vaccine study.
中文翻译:

生成具有成本效益的细胞系以支持原代人 B 细胞和单克隆中和抗体的高通量分离
人类病原体自然感染产生的人类单克隆抗体 (mAb) 的分离已被证明是一项强大的技术,有助于在分子水平上了解宿主对感染的反应。mAb 可以揭示病原体的易受攻击部位,并阐明抗原靶标的生物学功能。此外,单克隆抗体有可能直接用于治疗应用,例如被动递送以防止易感目标人群感染,以及治疗已感染。从疫苗试验中分离抗原特异性 B 细胞也有助于破译特定疫苗是否靶向所需的 B 细胞。已经开发了几种不同的方法来分离 mAb,但所有这些通常都是劳动密集型的,并导致不同程度的效率。在这里,我们描述了稳定表达 CD40 配体、白细胞介素 2 和白细胞介素 21 的具有成本效益的饲养细胞系的开发。将单个 B 细胞分选到一层受辐射的饲养细胞上可维持抗体的产生,从而允许以一种能够随后回收 B 细胞用于重组抗体克隆的方式对分泌的抗体进行功能性筛选。作为概念证明,我们表明这种方法可用于从 HPV 疫苗研究的参与者中分离 B 细胞,这些 B 细胞分泌可中和人乳头瘤病毒 (HPV) 的抗体。将单个 B 细胞分选到一层受辐射的饲养细胞上可维持抗体的产生,从而允许以一种能够随后回收 B 细胞用于重组抗体克隆的方式对分泌的抗体进行功能性筛选。作为概念证明,我们表明这种方法可用于从 HPV 疫苗研究的参与者中分离 B 细胞,这些 B 细胞分泌可中和人乳头瘤病毒 (HPV) 的抗体。将单个 B 细胞分选到一层受辐射的饲养细胞上可维持抗体的产生,从而允许以一种能够随后回收 B 细胞用于重组抗体克隆的方式对分泌的抗体进行功能性筛选。作为概念证明,我们表明这种方法可用于从 HPV 疫苗研究的参与者中分离 B 细胞,这些 B 细胞分泌可中和人乳头瘤病毒 (HPV) 的抗体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号