当前位置:
X-MOL 学术
›
Brain Stimul.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-invasive neuromodulation using rTMS and the Electromagnetic-Perceptive Gene (EPG) facilitates plasticity after nerve injury
Brain Stimulation ( IF 7.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.brs.2020.10.006 Carolina Cywiak 1 , Ryan C Ashbaugh 2 , Abigael C Metto 1 , Lalita Udpa 3 , Chunqi Qian 4 , Assaf A Gilad 5 , Mark Reimers 6 , Ming Zhong 7 , Galit Pelled 5
Brain Stimulation ( IF 7.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.brs.2020.10.006 Carolina Cywiak 1 , Ryan C Ashbaugh 2 , Abigael C Metto 1 , Lalita Udpa 3 , Chunqi Qian 4 , Assaf A Gilad 5 , Mark Reimers 6 , Ming Zhong 7 , Galit Pelled 5
Affiliation
|
BACKGROUND
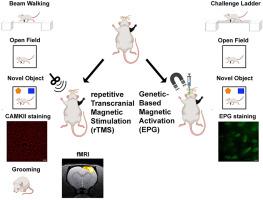
Twenty million Americans suffer from peripheral nerve injury. These patients often develop chronic pain and sensory dysfunctions. In the past decade, neuroimaging studies showed that these changes are associated with altered cortical excitation-inhibition balance and maladaptive plasticity. We tested if neuromodulation of the deprived sensory cortex could restore the cortical balance, and whether it would be effective in alleviating sensory complications. OBJECTIVE
We tested if non-invasive repetitive transcranial magnetic stimulation (rTMS) which induces neuronal excitability, and cell-specific magnetic activation via the Electromagnetic-perceptive gene (EPG) which is a novel gene that was identified and cloned from glass catfish and demonstrated to evoke neural responses when magnetically stimulated, can restore cortical excitability. METHODS
A rat model of forepaw denervation was used. rTMS was delivered every other day for 30 days, starting at the acute or at the chronic post-injury phase. A minimally-invasive neuromodulation via EPG was performed every day for 30 staring at the chronic phase. A battery of behavioral tests was performed in the days and weeks following limb denervation in EPG-treated rats, and behavioral tests, fMRI and immunochemistry were performed in rTMS-treated rats. RESULTS
The results demonstrate that neuromodulation significantly improved long-term mobility, decreased anxiety and enhanced neuroplasticity. The results identify that both acute and delayed rTMS intervention facilitated rehabilitation. Moreover, the results implicate EPG as an effective cell-specific neuromodulation approach. CONCLUSION
Together, these results reinforce the growing amount of evidence from human and animal studies that are establishing neuromodulation as an effective strategy to promote plasticity and rehabilitation.
中文翻译:

使用 rTMS 和电磁感知基因 (EPG) 的非侵入性神经调节促进神经损伤后的可塑性
背景两千万美国人患有外周神经损伤。这些患者经常出现慢性疼痛和感觉功能障碍。在过去十年中,神经影像学研究表明,这些变化与皮质兴奋-抑制平衡和适应不良可塑性的改变有关。我们测试了被剥夺的感觉皮层的神经调节是否可以恢复皮层平衡,以及它是否能有效减轻感觉并发症。目标我们测试了非侵入性重复经颅磁刺激 (rTMS) 是否通过电磁感知基因 (EPG) 诱导神经元兴奋性和细胞特异性磁激活,这是一种从玻璃鲶鱼中鉴定和克隆的新基因,并证明磁刺激时唤起神经反应,可以恢复皮质兴奋性。方法采用大鼠前爪去神经模型。从急性或慢性损伤后阶段开始,每隔一天进行一次 rTMS,持续 30 天。每天通过 EPG 进行微创神经调节,持续 30 年,凝视慢性期。在 EPG 治疗的大鼠肢体去神经支配后的几天和几周内进行了一系列行为测试,并在 rTMS 治疗的大鼠中进行了行为测试、fMRI 和免疫化学。结果 结果表明,神经调节可显着改善长期活动能力、减少焦虑并增强神经可塑性。结果表明,急性和延迟 rTMS 干预促进了康复。此外,结果表明 EPG 是一种有效的细胞特异性神经调节方法。结论 一起,
更新日期:2020-11-01
中文翻译:

使用 rTMS 和电磁感知基因 (EPG) 的非侵入性神经调节促进神经损伤后的可塑性
背景两千万美国人患有外周神经损伤。这些患者经常出现慢性疼痛和感觉功能障碍。在过去十年中,神经影像学研究表明,这些变化与皮质兴奋-抑制平衡和适应不良可塑性的改变有关。我们测试了被剥夺的感觉皮层的神经调节是否可以恢复皮层平衡,以及它是否能有效减轻感觉并发症。目标我们测试了非侵入性重复经颅磁刺激 (rTMS) 是否通过电磁感知基因 (EPG) 诱导神经元兴奋性和细胞特异性磁激活,这是一种从玻璃鲶鱼中鉴定和克隆的新基因,并证明磁刺激时唤起神经反应,可以恢复皮质兴奋性。方法采用大鼠前爪去神经模型。从急性或慢性损伤后阶段开始,每隔一天进行一次 rTMS,持续 30 天。每天通过 EPG 进行微创神经调节,持续 30 年,凝视慢性期。在 EPG 治疗的大鼠肢体去神经支配后的几天和几周内进行了一系列行为测试,并在 rTMS 治疗的大鼠中进行了行为测试、fMRI 和免疫化学。结果 结果表明,神经调节可显着改善长期活动能力、减少焦虑并增强神经可塑性。结果表明,急性和延迟 rTMS 干预促进了康复。此外,结果表明 EPG 是一种有效的细胞特异性神经调节方法。结论 一起,











































 京公网安备 11010802027423号
京公网安备 11010802027423号