Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-10-15 , DOI: 10.1016/j.bioorg.2020.104366 Mohamed A Yousef 1 , Ahmed M Ali 1 , Wael M El-Sayed 2 , Wesam S Qayed 1 , Hassan H A Farag 1 , Tarek Aboul-Fadl 1
|
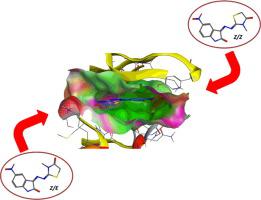
In recent years, cell cycle and checkpoint pathways regulation are offering new therapeutic approaches against cancer. Isatin, is a well exploited scaffold in the anticancer domain. Accordingly, the current work describes the design and synthesis of two series of (Z)-3-substituted-2-(((E/Z)-5-substituted-2-oxo-1-substituted-indolin-3-ylidene)hydrazinylidene)-thiazolidin-4-ones, 4(a-s) and (E/Z)-1-substituted-3-(((Z)-3-substituted-4-methylthiazol-2(3H)-ylidene)hydrazineylidene)-5-substituted-indolin-2-ones, 5(a-s). The structures of the synthesized molecules were confirmed by spectral and elemental methods of analyses. Pure diastereomers were further identified with 1H-1H-NOESY and confirmed with X-ray crystallography. The target compounds were tested in vitro for their cytotoxicity against three human epithelial cell lines, liver (HepG2), breast (MCF-7), and colon (HT-29) in addition to the diploid human normal cells (WI-38) compared to doxorubicin as a reference drug. Variable cytotoxic effects (IC50 3.29–100 µmol) were reported on the three cancer cell lines with pronounced selectivity compared to the normal one WI-38. The potency of the most active compounds, 4o, 4s, 5e, 5f, 5l, 5m and 5o (IC50 3.29–9.92 µmol), in both series associated with the (Z) configurations of N = thiazolidin/ene or one, however, the configuration of the N = isatin moiety seemed to be of no importance to the activity. The tested compounds were grouped for their possible mechanism of action into 4 categories. Compound 4o with no apparent effect on all genes examined. Compounds 4s and 5o affected all genes investigated and seem to have multiple cellular targets; induced the expression of p53 and caspases, and downregulated that of CDK1. Compounds 5l and 5m directly elevated the expression of initiator and effector caspases without going through p53 pathway. Finally, compounds 5e and 5f elevated the expression of p53 and inhibited CDK1. Compounds 4s, 5e, 5f, 5l, 5m, and 5o caused a significant elevation in the activity of cleaved caspase 3 as well. Docking studies on CDK1 revealed that the active molecules bind to the tested enzyme by the same manner of the co-crystallized ligands and the isatin-thiazoldinone/ene scaffold is essential for binding of these molecules.
中文翻译:

靶向细胞周期检查点通路的新型靛红衍生物的设计和合成作为潜在的抗癌剂
近年来,细胞周期和检查点通路的调节正在为癌症提供新的治疗方法。靛红是一种在抗癌领域得到充分利用的支架。因此,目前的工作描述了两个系列(Z)-3-取代-2-(((E / Z)-5-取代-2-氧代-1-取代-二氢吲哚-3-亚基)的设计和合成亚肼基)-噻唑烷-4-酮,4(as) 和 (E/Z)-1-取代-3-(((Z)-3-取代-4-甲基噻唑-2(3H)-亚基)亚肼基)- 5-取代-二氢吲哚-2-ones,5(as)。通过光谱和元素分析方法证实了合成分子的结构。用1 H- 1 H-NOESY进一步鉴定纯的非对映异构体,并用 X 射线晶体学证实。目标化合物在体外测试与多柔比星作为参考药物相比,它们对三种人类上皮细胞系、肝脏 (HepG2)、乳腺 (MCF-7) 和结肠 (HT-29) 以及二倍体人类正常细胞 (WI-38) 的细胞毒性。与正常的一种 WI-38 相比,三种癌细胞系报告了可变的细胞毒性作用 (IC 50 3.29–100 µmol),具有明显的选择性。最活跃的化合物 4o、4s、5e、5f、5l、5m 和 5o 的效力(IC 503.29–9.92 µmol),在与 N = 噻唑烷/烯的 (Z) 构型相关的两个系列中,N = 靛红部分的构型似乎对活性无关紧要。测试的化合物根据其可能的作用机制分为 4 类。化合物 4o 对所有检查的基因没有明显影响。化合物 4s 和 5o 影响所有研究的基因,似乎有多个细胞靶点;诱导 p53 和 caspase 的表达,并下调 CDK1 的表达。化合物 5l 和 5m 不通过 p53 途径直接提高起始和效应半胱天冬酶的表达。最后,化合物 5e 和 5f 提高了 p53 的表达并抑制了 CDK1。化合物 4s、5e、5f、5l、5m 和 5o 也导致裂解的半胱天冬酶 3 的活性显着升高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号