当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical investigations of electrochemical CO2 reduction by transition metals anchored on CNTs
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-10-14 , DOI: 10.1039/d0se01127d Chengcheng Ao 1, 2, 3, 4 , Wei Zhao 1, 2, 3, 4 , Shanshan Ruan 1, 2, 3, 4 , Siyu Qian 1, 2, 3, 4 , Yi Liu 1, 2, 3, 4 , Lei Wang 1, 2, 3, 4 , Lidong Zhang 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-10-14 , DOI: 10.1039/d0se01127d Chengcheng Ao 1, 2, 3, 4 , Wei Zhao 1, 2, 3, 4 , Shanshan Ruan 1, 2, 3, 4 , Siyu Qian 1, 2, 3, 4 , Yi Liu 1, 2, 3, 4 , Lei Wang 1, 2, 3, 4 , Lidong Zhang 1, 2, 3, 4, 5
Affiliation
|
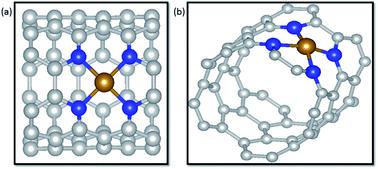
Transition metals supported on nitrogen doped carbon materials are a class of promising electrochemical catalysts toward electrochemical CO2 reduction reactions (CO2RR) that have exhibited excellent catalytic performance. Herein, M–N4 (M = Fe, Co and Ni) coordination structures embedded in carbon nanotubes (CNTs) were constructed to explore detailed mechanisms as electrocatalysts for CO2RR via density functional theory (DFT) calculations. Nitrogen atoms of coordination structures rather than only single transition metal atoms were demonstrated to be effective active sites toward CO2RR. For two possible pathways of the first step, forming *COOH or *OCHO, according to the catalytic activity of nitrogen atoms to the H atom, *COOH generation was facilitated in terms of kinetics over *OCHO in three systems. Meantime, it is suggested that the products of electrochemical CO2RR on the three catalysts are heavily dependent on the interaction of CO and the catalysts. Ni–N4/CNT exhibits a considerable selectivity to CO due to the weak interaction of CO and the substrates with a limiting potential of 1.79 V. On the contrary, CO prefers to remain on Fe–N4/CNT due to the strong binding energy of CO adsorbed on Fe–N4/CNT. Then the CO of the Fe–N4/CNT system undergoes further hydrogenation to produce CH3OH and CH4 at the same limiting potential of 0.68 V, indicating that there is no distinct selectivity in forming CH3OH and CH4. Nevertheless, the limiting potentials of CO, CH3OH and CH4 on Co–N4/CNT are totally different, 0.43 V, 0.56 V and 0.87 V, respectively. In particular, CO2RR to CO on Co–N4/CNT has the lowest potential limit. Thus, Co–N4/CNT has a considerable potential as the catalyst for electrochemical CO2RR. In addition, the catalysts of Ni single atoms, unsaturated coordination with nitrogen atoms, edge-anchored on CNTs were investigated for their activity for the CO2RR. Our comprehensive understanding of M–N4/CNT materials may be instructive and meaningful to design advanced catalysts from nonprecious metals for electrochemical CO2RR.
中文翻译:

固定在CNT上的过渡金属对电化学CO2还原的理论研究
负载在氮掺杂碳材料上的过渡金属是一类有前途的电化学催化剂,可用于表现出出色催化性能的电化学CO 2还原反应(CO 2 RR)。本文中,构建了嵌入碳纳米管(CNT)中的M–N 4(M = Fe,Co和Ni)配位结构,以通过密度泛函理论(DFT)计算来探索作为CO 2 RR电催化剂的详细机理。配位结构的氮原子而不是单个过渡金属原子被证明是有效的CO 2活性位点RR。对于第一步的两个可能的途径,形成* COOH或* OCHO,根据氮原子对H原子的催化活性,在动力学上,在三个系统中* COOH的生成优于* OCHO。同时,提出三种催化剂上的电化学CO 2 RR产物在很大程度上取决于CO和催化剂的相互作用。Ni–N 4 / CNT对CO表现出相当大的选择性,这是因为CO与底物之间的弱相互作用,极限电位为1.79V。相反,由于结合力强,CO倾向于保留在Fe–N 4 / CNT上CO吸附在Fe–N 4 / CNT上的能量。然后是Fe–N 4的CO/ CNT系统进一步加氢以在相同的极限电势0.68 V下生成CH 3 OH和CH 4,表明形成CH 3 OH和CH 4时没有明显的选择性。但是,CO,CH 3 OH和CH 4在Co–N 4 / CNT上的极限电位完全不同,分别为0.43 V,0.56 V和0.87V。特别是,Co–N 4 / CNT上的CO 2 RR对CO的最低电位极限。因此,Co–N 4 / CNT具有作为电化学CO 2催化剂的巨大潜力。RR。另外,研究了碳纳米管边缘固定在碳纳米管上的Ni单原子,与氮原子不饱和配位的催化剂对CO 2 RR的活性。我们对M–N 4 / CNT材料的全面理解对于用非贵金属设计用于电化学CO 2 RR的高级催化剂可能具有指导意义和意义。
更新日期:2020-11-03
中文翻译:

固定在CNT上的过渡金属对电化学CO2还原的理论研究
负载在氮掺杂碳材料上的过渡金属是一类有前途的电化学催化剂,可用于表现出出色催化性能的电化学CO 2还原反应(CO 2 RR)。本文中,构建了嵌入碳纳米管(CNT)中的M–N 4(M = Fe,Co和Ni)配位结构,以通过密度泛函理论(DFT)计算来探索作为CO 2 RR电催化剂的详细机理。配位结构的氮原子而不是单个过渡金属原子被证明是有效的CO 2活性位点RR。对于第一步的两个可能的途径,形成* COOH或* OCHO,根据氮原子对H原子的催化活性,在动力学上,在三个系统中* COOH的生成优于* OCHO。同时,提出三种催化剂上的电化学CO 2 RR产物在很大程度上取决于CO和催化剂的相互作用。Ni–N 4 / CNT对CO表现出相当大的选择性,这是因为CO与底物之间的弱相互作用,极限电位为1.79V。相反,由于结合力强,CO倾向于保留在Fe–N 4 / CNT上CO吸附在Fe–N 4 / CNT上的能量。然后是Fe–N 4的CO/ CNT系统进一步加氢以在相同的极限电势0.68 V下生成CH 3 OH和CH 4,表明形成CH 3 OH和CH 4时没有明显的选择性。但是,CO,CH 3 OH和CH 4在Co–N 4 / CNT上的极限电位完全不同,分别为0.43 V,0.56 V和0.87V。特别是,Co–N 4 / CNT上的CO 2 RR对CO的最低电位极限。因此,Co–N 4 / CNT具有作为电化学CO 2催化剂的巨大潜力。RR。另外,研究了碳纳米管边缘固定在碳纳米管上的Ni单原子,与氮原子不饱和配位的催化剂对CO 2 RR的活性。我们对M–N 4 / CNT材料的全面理解对于用非贵金属设计用于电化学CO 2 RR的高级催化剂可能具有指导意义和意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号