当前位置:
X-MOL 学术
›
J. Mater. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapochromism induced by intermolecular electron transfer coupled with hydrogen-bond formation in zinc dithiolene complex
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2020-10-14 , DOI: 10.1039/d0tc04280c So Yokomori 1, 2, 3, 4 , Shun Dekura 1, 2, 3, 4 , Tomoko Fujino 1, 2, 3, 4 , Mitsuaki Kawamura 1, 2, 3, 4 , Taisuke Ozaki 1, 2, 3, 4 , Hatsumi Mori 1, 2, 3, 4
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2020-10-14 , DOI: 10.1039/d0tc04280c So Yokomori 1, 2, 3, 4 , Shun Dekura 1, 2, 3, 4 , Tomoko Fujino 1, 2, 3, 4 , Mitsuaki Kawamura 1, 2, 3, 4 , Taisuke Ozaki 1, 2, 3, 4 , Hatsumi Mori 1, 2, 3, 4
Affiliation
|
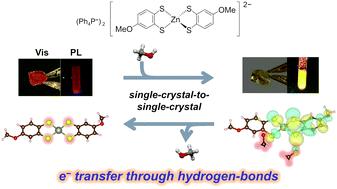
Hydrogen bonding (H-bonding) has long been used for structural regulations. Recently, some examples have been reported where electrical conductivity and magnetism were switched based on direct modulation of electronic states through H-bond. If the application of this strategy is realized to a wide range of functionalities, including optical properties, it will greatly expand the design criteria of functional molecular materials. Here, we report a novel zinc dithiolene complex crystal, which exhibits a new vapochromism induced by electron transfer through H-bonds. This crystal exhibits changes in both visible-light absorption and photoluminescence properties with single-crystal-to-single-crystal (SCSC) transformations caused by methanol or water vapor absorption. Single-crystal structural analysis revealed that the zinc dithiolene complex formed H-bonds with the vapor molecules. Importantly, first-principles calculations based on the periodic crystal structures indicated that the vapochromism in this crystal was solely induced by H-bond formation, not by structural changes. Detailed investigations of the energies and shapes of the crystal orbitals as well as Mulliken population analysis revealed that electron transfer through the H-bonds caused vapochromism in this crystal. The findings provide important insights into the material designs of novel vapochromic materials as well as other stimuli-responsive materials considering the intermolecular H-bonds in the solid-state.
中文翻译:

分子间电子转移与氢键形成二硫代锌配合物引起的气相致变色
氢键(H键)长期以来一直用于结构法规。最近,已经报道了一些示例,其中基于通过H键对电子态的直接调制来切换电导率和磁性。如果将这种策略的应用实现到包括光学性质在内的广泛功能,它将大大扩展功能分子材料的设计标准。在这里,我们报告了一种新型的二硫代锌络合物晶体,它表现出通过电子通过H键转移引起的新的变色。该晶体在甲醇或水蒸气吸收引起的单晶至单晶(SCSC)转换中表现出可见光吸收和光致发光特性的变化。单晶结构分析表明二硫代锌配合物与蒸汽分子形成氢键。重要的是,基于周期性晶体结构的第一性原理计算表明,该晶体中的气相致变色现象完全是由氢键形成引起的,而不是由结构变化引起的。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。基于周期性晶体结构的第一性原理计算表明,该晶体中的气相致变色现象仅由氢键形成引起,而不是由结构变化引起。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。基于周期性晶体结构的第一性原理计算表明,该晶体中的气相致变色现象仅由氢键形成引起,而不是由结构变化引起。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。
更新日期:2020-10-14
中文翻译:

分子间电子转移与氢键形成二硫代锌配合物引起的气相致变色
氢键(H键)长期以来一直用于结构法规。最近,已经报道了一些示例,其中基于通过H键对电子态的直接调制来切换电导率和磁性。如果将这种策略的应用实现到包括光学性质在内的广泛功能,它将大大扩展功能分子材料的设计标准。在这里,我们报告了一种新型的二硫代锌络合物晶体,它表现出通过电子通过H键转移引起的新的变色。该晶体在甲醇或水蒸气吸收引起的单晶至单晶(SCSC)转换中表现出可见光吸收和光致发光特性的变化。单晶结构分析表明二硫代锌配合物与蒸汽分子形成氢键。重要的是,基于周期性晶体结构的第一性原理计算表明,该晶体中的气相致变色现象完全是由氢键形成引起的,而不是由结构变化引起的。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。基于周期性晶体结构的第一性原理计算表明,该晶体中的气相致变色现象仅由氢键形成引起,而不是由结构变化引起。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。基于周期性晶体结构的第一性原理计算表明,该晶体中的气相致变色现象仅由氢键形成引起,而不是由结构变化引起。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。对晶体轨道的能量和形状的详细研究以及Mulliken种群分析表明,通过H键的电子转移导致该晶体发生气相致变色。该发现为新型气相致变色材料以及考虑到固态分子间氢键的其他刺激响应材料的材料设计提供了重要的见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号