当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Seawater on the Morphological Evolution and the Microchemistry of Hydration Products of Tricalcium Silicates (C3S)
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-10-14 , DOI: 10.1021/acssuschemeng.0c04440 Sarah Abduljabbar Yaseen 1, 2 , Ghadah Abdaljabar Yiseen 3 , Chi Sun Poon 1 , Zongjin Li 4
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-10-14 , DOI: 10.1021/acssuschemeng.0c04440 Sarah Abduljabbar Yaseen 1, 2 , Ghadah Abdaljabar Yiseen 3 , Chi Sun Poon 1 , Zongjin Li 4
Affiliation
|
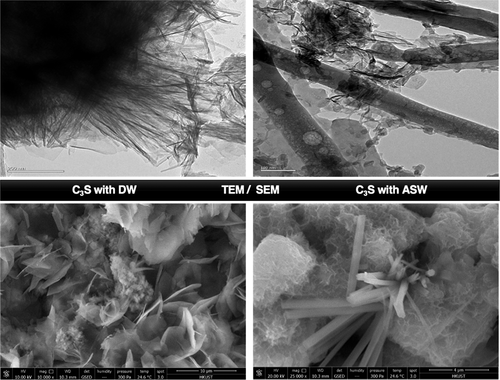
The current work investigates the influence of seawater on morphological properties during the hydration process of tricalcium silicate (C3S) at 3, 7, 14, and 28 days to better understand the effect of salinity (highly soluble salts) of seawater on the microstructural evolution of hydration products. The mechanism of the chemical reaction of highly soluble salts, e.g., Na2SO4 and CaCl2, with hydrated C3S was also demonstrated. The presence of highly soluble salts in seawater accelerates the hydration of C3S significantly due to releasing a significant amount of Ca2+ ions from the hydrated C3S (as CH and CSH), which participated in the chemical reaction to produce a certain amount of gypsum crystals that was more than that in distilled water, which has been shown by SEM characterization. TEM analysis revealed the growth of sharp rod-like CaSO4·2H2O crystals together with some thin and tiny wrinkled CSH that formed. Seawater promotes the hydration of C3S, pointed out by the expedited heat flow and raised heat of hydration. FTIR spectroscopy has been used to characterize and observe the dynamics of variation in the formation of calcium silicate hydrate (CSH), portlandite (CH), and gypsum (Gy) throughout the hydration process of C3S with seawater in comparison with distilled water. XRD analysis revealed that the peak intensities of the portlandite and gypsum of the hydrated C3S in seawater are higher than the comparable peaks in distilled water.
中文翻译:

海水对硅酸三钙(C 3 S)形态演变和水合产物微观化学的影响
当前的工作研究了在三,七,十四和二十八天硅酸三钙(C 3 S)水化过程中海水对形态特性的影响,以更好地了解海水盐度(高可溶性盐)对微观结构的影响。水合产物的演变。还证明了高可溶性盐(例如Na 2 SO 4和CaCl 2)与水合C 3 S的化学反应机理。海水中高度溶解的盐的存在,由于从水合C 3中释放出大量的Ca 2+离子,从而大大促进了C 3 S的水合S(作为CH和CSH)参与化学反应,生成一定数量的石膏晶体,该晶体比蒸馏水中的石膏晶体多,这已通过SEM表征了。TEM分析显示出尖锐的棒状CaSO 4 ·2H 2 O晶体的生长以及形成的细而细的皱纹CSH。海水促进了C 3 S的水合作用,这是由加速的热流和水合热量升高所指出的。FTIR光谱已用于表征和观察在整个C 3的水合过程中硅酸钙水合物(CSH),硅酸盐(CH)和石膏(Gy)形成过程中的变化动态。海水中的S与蒸馏水相比。XRD分析表明,海水中水合C 3 S的钙钛矿和石膏的峰强度高于蒸馏水中的可比峰。
更新日期:2020-10-26
中文翻译:

海水对硅酸三钙(C 3 S)形态演变和水合产物微观化学的影响
当前的工作研究了在三,七,十四和二十八天硅酸三钙(C 3 S)水化过程中海水对形态特性的影响,以更好地了解海水盐度(高可溶性盐)对微观结构的影响。水合产物的演变。还证明了高可溶性盐(例如Na 2 SO 4和CaCl 2)与水合C 3 S的化学反应机理。海水中高度溶解的盐的存在,由于从水合C 3中释放出大量的Ca 2+离子,从而大大促进了C 3 S的水合S(作为CH和CSH)参与化学反应,生成一定数量的石膏晶体,该晶体比蒸馏水中的石膏晶体多,这已通过SEM表征了。TEM分析显示出尖锐的棒状CaSO 4 ·2H 2 O晶体的生长以及形成的细而细的皱纹CSH。海水促进了C 3 S的水合作用,这是由加速的热流和水合热量升高所指出的。FTIR光谱已用于表征和观察在整个C 3的水合过程中硅酸钙水合物(CSH),硅酸盐(CH)和石膏(Gy)形成过程中的变化动态。海水中的S与蒸馏水相比。XRD分析表明,海水中水合C 3 S的钙钛矿和石膏的峰强度高于蒸馏水中的可比峰。







































 京公网安备 11010802027423号
京公网安备 11010802027423号