当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Hazards for Autocatalysis and Stability in CSTR: Decomposition of Solution from Nitrolysis of Hexamethylenetetramine
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2020-10-14 , DOI: 10.1002/prep.202000087 Qiang Xie 1 , Luyao Zhang 2 , Xianhan Yu 2 , Wei Liu 1 , Fei Jiang 1 , Jie He 1 , Yaoxuan Zhang 3 , Houhe Chen 1
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2020-10-14 , DOI: 10.1002/prep.202000087 Qiang Xie 1 , Luyao Zhang 2 , Xianhan Yu 2 , Wei Liu 1 , Fei Jiang 1 , Jie He 1 , Yaoxuan Zhang 3 , Houhe Chen 1
Affiliation
|
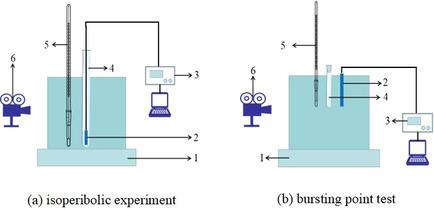
Nitrolysis solution is a kind of spent acid, obtained after the nitration and ripening of hexamethylenetetramine (HA). Its decomposition is generally spontaneous and hazardous. Therefore, the thermal hazards of a real nitrolysis solution were studied here. The prepared nitrolysis solution was analysed by isoperibolic experiments, differential thermal analysis (DTA), accelerating rate calorimetry (ARC), and a bursting point test. We employed non‐linear regression and ordinary differential equation (ODE) fitting methods to obtain kinetic parameters, such as 120.73 kJ mol−1 of the activation energy (E), 36.388 s−1 of the natural logarithm of the pre‐exponential factor (lnA), 1.155 of the reaction order, and 0.6615 of the catalytic index. Based on these kinetic parameters, thermal safety parameters were obtained to evaluate the hazards of a real nitrolysis solution from four aspects: severity, thermal stability, process hazards, and thermal sensitivity. The results indicate critical severity, high process hazards and thermal sensitivity, and very low thermal stability. The hazards due to real nitrolysis solution are higher than those of nitric ester explosives and azos. Further, the value of the initial mass ratio between the concentrated nitric acid and HA could severely pose safety risks during the nitration process. Additionally, the sensitivity analyses of the four process parameters (space‐time of CSTR, τCSTR; feeding temperature, T0; ambient temperature, Ta; specific cooling capacity, hS/V) were performed under the critical conditions for thermal stability in a continuous stirred tank reactor (CSTR). The suited margin of Ta could be 333.15–342.81 K with other determined process parameters.
中文翻译:

CSTR中自催化和稳定性的热危险:六亚甲基四胺硝化后溶液的分解
硝化溶液是一种废酸,在六亚甲基四胺(HA)硝化和成熟后获得。它的分解通常是自发的和危险的。因此,这里研究了真正的硝解溶液的热危害。通过等代谢实验,差热分析(DTA),加速量热法(ARC)和爆点测试对制备的硝化溶液进行分析。我们采用非线性回归和常微分方程(ODE)拟合方法获得动力学参数,例如活化能(E)为120.73 kJ mol -1,指数前自然对数的自然对数为36.388 s -1( LN一),反应顺序的1.155和催化指数的0.6615。基于这些动力学参数,获得了热安全参数,以从四个方面评估真实硝解溶液的危害:严重性,热稳定性,过程危害和热敏感性。结果表明严重程度高,过程危险性和热敏感性高,并且热稳定性非常低。实际硝解溶液的危害要高于硝酸酯炸药和偶氮的危害。此外,浓硝酸和HA之间的初始质量比的值可能严重地在硝化过程中带来安全隐患。此外,该灵敏度的四个处理参数(CSTR的空间-时间,的分析τ CSTR ;将温度,Ť 0; 环境温度,T a;在连续搅拌釜式反应器(CSTR)的热稳定性的关键条件下进行了特定的冷却能力(hS / V)。使用其他确定的过程参数,T a的合适裕度可以为333.15–342.81K。
更新日期:2020-12-07
中文翻译:

CSTR中自催化和稳定性的热危险:六亚甲基四胺硝化后溶液的分解
硝化溶液是一种废酸,在六亚甲基四胺(HA)硝化和成熟后获得。它的分解通常是自发的和危险的。因此,这里研究了真正的硝解溶液的热危害。通过等代谢实验,差热分析(DTA),加速量热法(ARC)和爆点测试对制备的硝化溶液进行分析。我们采用非线性回归和常微分方程(ODE)拟合方法获得动力学参数,例如活化能(E)为120.73 kJ mol -1,指数前自然对数的自然对数为36.388 s -1( LN一),反应顺序的1.155和催化指数的0.6615。基于这些动力学参数,获得了热安全参数,以从四个方面评估真实硝解溶液的危害:严重性,热稳定性,过程危害和热敏感性。结果表明严重程度高,过程危险性和热敏感性高,并且热稳定性非常低。实际硝解溶液的危害要高于硝酸酯炸药和偶氮的危害。此外,浓硝酸和HA之间的初始质量比的值可能严重地在硝化过程中带来安全隐患。此外,该灵敏度的四个处理参数(CSTR的空间-时间,的分析τ CSTR ;将温度,Ť 0; 环境温度,T a;在连续搅拌釜式反应器(CSTR)的热稳定性的关键条件下进行了特定的冷却能力(hS / V)。使用其他确定的过程参数,T a的合适裕度可以为333.15–342.81K。










































 京公网安备 11010802027423号
京公网安备 11010802027423号