当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning oxygen reduction activity on chromia surface via alloying: a DFT study
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-10-13 , DOI: 10.1002/asia.202000997 Man‐Fai Ng 1 , Daniel John Blackwood 2 , Hongmei Jin 1 , Teck Leong Tan 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-10-13 , DOI: 10.1002/asia.202000997 Man‐Fai Ng 1 , Daniel John Blackwood 2 , Hongmei Jin 1 , Teck Leong Tan 1
Affiliation
|
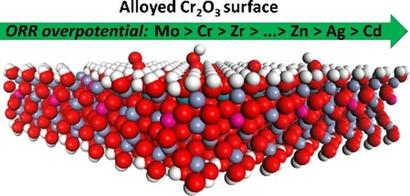
Economical electro‐catalysts for the oxygen reduction reaction (ORR) are highly desirable for a range of advance energy storage technologies. Chromium compounds have been suggested as one possible source of non‐precious metal based catalysts for oxygen reduction reaction (ORR), especially chromia (Cr2O3) which is the most stable form of Cr oxide at room temperature. Using density functional theory+U calculations, we investigate the 4‐electron ORR on the hydroxylated Cr2O3 surfaces alloyed with 17 different transition metals. On the one hand, we find that the ORR overpotential is lower when the Cr2O3 surface alloyed with elements towards the end of both the first and second rows of transition metals. Among these elements, Cd alloyed Cr2O3 surface is found to promote the ORR the most, but due to its high toxicity and price it loses out to Zn as the recommended alloyant. On the other hand, we find that the ORR overpotential is generally higher and less varied on the Cr2O3 surface alloyed with the early‐to‐mid row transition metal elements (e. g. Zr, Ti). As Cr2O3 is also a major component in the passive film on stainless steels, where a low ORR rate is desirable to reduce the impact of localized corrosion. This implies that alloying with early‐to‐mid row transition elements could be beneficial to stainless steels. The difference in oxygen reduction activity is attributed to the tendency of forming stable ORR intermediates during the oxygen reduction process.
中文翻译:

通过合金调整氧化铬表面的氧还原活性:DFT研究
对于一系列先进的储能技术,氧还原反应(ORR)的经济型电子催化剂是非常理想的。有人建议将铬化合物用作氧还原反应(ORR)的非贵金属基催化剂的一种可能来源,尤其是铬(Cr 2 O 3),它是室温下最稳定的氧化铬形式。使用密度泛函理论+ U计算,我们研究了与17种不同过渡金属合金化的羟基化Cr 2 O 3表面上的4电子ORR 。一方面,我们发现当Cr 2 O 3时,ORR过电势较低在第一和第二排过渡金属的末端都与元素形成合金表面。在这些元素中,发现Cd合金化的Cr 2 O 3表面最大程度地促进了ORR,但由于其高毒性和价格优势,它被推荐的合金元素Zn取代。另一方面,我们发现与早期至中行过渡金属元素(例如Zr,Ti)合金化的Cr 2 O 3表面,ORR超电势通常较高而变化较小。作为Cr 2 O 3它也是不锈钢钝化膜中的主要成分,需要低ORR率以减少局部腐蚀的影响。这意味着与早期到中期排过渡元素的合金化可能对不锈钢有利。氧还原活性的差异归因于在氧还原过程中形成稳定的ORR中间体的趋势。
更新日期:2020-12-01
中文翻译:

通过合金调整氧化铬表面的氧还原活性:DFT研究
对于一系列先进的储能技术,氧还原反应(ORR)的经济型电子催化剂是非常理想的。有人建议将铬化合物用作氧还原反应(ORR)的非贵金属基催化剂的一种可能来源,尤其是铬(Cr 2 O 3),它是室温下最稳定的氧化铬形式。使用密度泛函理论+ U计算,我们研究了与17种不同过渡金属合金化的羟基化Cr 2 O 3表面上的4电子ORR 。一方面,我们发现当Cr 2 O 3时,ORR过电势较低在第一和第二排过渡金属的末端都与元素形成合金表面。在这些元素中,发现Cd合金化的Cr 2 O 3表面最大程度地促进了ORR,但由于其高毒性和价格优势,它被推荐的合金元素Zn取代。另一方面,我们发现与早期至中行过渡金属元素(例如Zr,Ti)合金化的Cr 2 O 3表面,ORR超电势通常较高而变化较小。作为Cr 2 O 3它也是不锈钢钝化膜中的主要成分,需要低ORR率以减少局部腐蚀的影响。这意味着与早期到中期排过渡元素的合金化可能对不锈钢有利。氧还原活性的差异归因于在氧还原过程中形成稳定的ORR中间体的趋势。









































 京公网安备 11010802027423号
京公网安备 11010802027423号