当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper‐Catalyzed Asymmetric Coupling of Allenyl Radicals with Terminal Alkynes to Access Tetrasubstituted Allenes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-14 , DOI: 10.1002/anie.202013022 Xiao‐Yang Dong 1 , Tian‐Ya Zhan 1 , Sheng‐Peng Jiang 1 , Xiao‐Dong Liu 1 , Liu Ye 2 , Zhong‐Liang Li 2 , Qiang‐Shuai Gu 2 , Xin‐Yuan Liu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-14 , DOI: 10.1002/anie.202013022 Xiao‐Yang Dong 1 , Tian‐Ya Zhan 1 , Sheng‐Peng Jiang 1 , Xiao‐Dong Liu 1 , Liu Ye 2 , Zhong‐Liang Li 2 , Qiang‐Shuai Gu 2 , Xin‐Yuan Liu 1
Affiliation
|
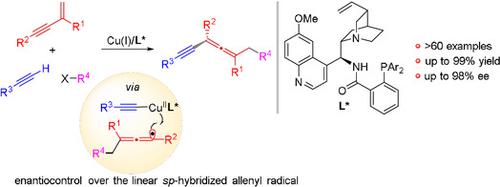
In contrast to the wealth of asymmetric transformations for generating central chirality from alkyl radicals, the enantiocontrol over the allenyl radicals for forging axial chirality represents an uncharted domain. The challenge arises from the unique elongated linear configuration of the allenyl radicals that necessitates the stereo‐differentiation of remote motifs away from the radical reaction site. We herein describe a copper‐catalyzed asymmetric radical 1,4‐carboalkynylation of 1,3‐enynes via the coupling of allenyl radicals with terminal alkynes, providing diverse synthetically challenging tetrasubstituted chiral allenes. A chiral N,N,P‐ligand is crucial for both the reaction initiation and the enantiocontrol over the highly reactive allenyl radicals. The reaction features a broad substrate scope, covering a variety of (hetero)aryl and alkyl alkynes and 1,3‐enynes as well as radical precursors with excellent functional group tolerance.
中文翻译:

铜催化的烯丙基自由基与末端炔烃的不对称偶联以访问四取代的烯丙基
与大量用于从烷基自由基产生中心手性的不对称转变相反,对用于锻造轴向手性的烯基的对映体控制代表了一个未知领域。挑战来自于烯基自由基独特的细长线性构型,这使得远离自由基反应位点的远程基序立体区分成为必要。我们在本文中描述了通过烯丙基与末端炔烃的偶联,铜催化的1,3-烯炔烃的不对称基团1,4-碳炔基化反应,从而提供了各种合成上具有挑战性的四取代手性烯丙基。手性N,N,P-配体对于高反应性烯基自由基的反应引发和对映体控制均至关重要。该反应具有广泛的底物范围,
更新日期:2020-10-14
中文翻译:

铜催化的烯丙基自由基与末端炔烃的不对称偶联以访问四取代的烯丙基
与大量用于从烷基自由基产生中心手性的不对称转变相反,对用于锻造轴向手性的烯基的对映体控制代表了一个未知领域。挑战来自于烯基自由基独特的细长线性构型,这使得远离自由基反应位点的远程基序立体区分成为必要。我们在本文中描述了通过烯丙基与末端炔烃的偶联,铜催化的1,3-烯炔烃的不对称基团1,4-碳炔基化反应,从而提供了各种合成上具有挑战性的四取代手性烯丙基。手性N,N,P-配体对于高反应性烯基自由基的反应引发和对映体控制均至关重要。该反应具有广泛的底物范围,











































 京公网安备 11010802027423号
京公网安备 11010802027423号