当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Meaning of “Collision Rate Constants” for Ion-Molecule Reactions: Association of Hydrogen Atoms with C6H5+ and Small Alkyl Radicals with C7H7+ Ions
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.ijms.2020.116455 S.G. Ard , C.J. Cobos , A.I. Maergoiz , N.S. Shuman , J. Troe , A.A. Viggiano
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.ijms.2020.116455 S.G. Ard , C.J. Cobos , A.I. Maergoiz , N.S. Shuman , J. Troe , A.A. Viggiano
|
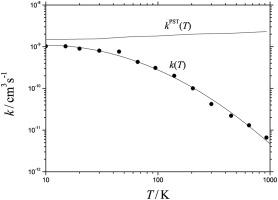
Abstract Limiting high pressure rate constants for the association of H with C6H5+ and of CH3, C2H5, and n-C3H7 radicals with C7H7+ molecular ions are analyzed in terms of “rigidity factors” arising from the anisotropy of the interaction potentials and “collision rate constants” in the absence of anisotropy (the latter corresponding to phase space theory, PST). Model calculations based on ab initio potential energy surfaces show that the PST rate constants k PST(T) exceed collision rate constants from conventional ion-molecule capture theory (in this case given by Langevin rate constants kL). They can be represented by k PST(T) = kL + kh.sph. where kh.sph. denotes hard-sphere collision numbers with collision radii r0. The r0 derived from the modelled k PST(T) are related to properties of the interaction potentials. Applications to other ion-molecule reactions are proposed.
中文翻译:

关于离子分子反应“碰撞速率常数”的含义:氢原子与 C6H5+ 和小烷基与 C7H7+ 离子的缔合
摘要 根据相互作用势的各向异性和“碰撞速率常数”产生的“刚性因子”分析了 H 与 C6H5+ 结合以及 CH3、C2H5 和 n-C3H7 自由基与 C7H7+ 分子离子缔合的极限高压速率常数。 ”在没有各向异性的情况下(后者对应于相空间理论,PST)。基于 ab initio 势能面的模型计算表明,PST 速率常数 k PST(T) 超过了来自传统离子分子捕获理论的碰撞速率常数(在这种情况下由朗之万速率常数 kL 给出)。它们可以表示为 k PST(T) = kL + kh.sph。哪里 kh.sph。表示碰撞半径为 r0 的硬球碰撞数。从建模的 k PST(T) 导出的 r0 与相互作用势的特性有关。
更新日期:2020-12-01
中文翻译:

关于离子分子反应“碰撞速率常数”的含义:氢原子与 C6H5+ 和小烷基与 C7H7+ 离子的缔合
摘要 根据相互作用势的各向异性和“碰撞速率常数”产生的“刚性因子”分析了 H 与 C6H5+ 结合以及 CH3、C2H5 和 n-C3H7 自由基与 C7H7+ 分子离子缔合的极限高压速率常数。 ”在没有各向异性的情况下(后者对应于相空间理论,PST)。基于 ab initio 势能面的模型计算表明,PST 速率常数 k PST(T) 超过了来自传统离子分子捕获理论的碰撞速率常数(在这种情况下由朗之万速率常数 kL 给出)。它们可以表示为 k PST(T) = kL + kh.sph。哪里 kh.sph。表示碰撞半径为 r0 的硬球碰撞数。从建模的 k PST(T) 导出的 r0 与相互作用势的特性有关。









































 京公网安备 11010802027423号
京公网安备 11010802027423号