当前位置:
X-MOL 学术
›
Atmos. Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron speciation in particulate matter (PM2.5) from urban Los Angeles using spectro-microscopy methods
Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.atmosenv.2020.117988 Ajith Pattammattel 1 , Valerie J Leppert 2 , Paul Aronstein 3 , Matthew Robinson 2 , Amirhosein Mousavi 4 , Constantinos Sioutas 4 , Henry Jay Forman 5 , Peggy A O'Day 1, 3
Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.atmosenv.2020.117988 Ajith Pattammattel 1 , Valerie J Leppert 2 , Paul Aronstein 3 , Matthew Robinson 2 , Amirhosein Mousavi 4 , Constantinos Sioutas 4 , Henry Jay Forman 5 , Peggy A O'Day 1, 3
Affiliation
|
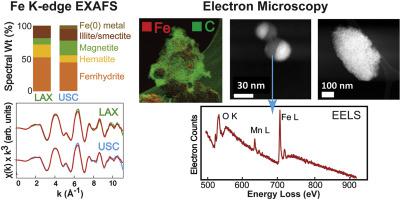
The speciation, oxidation states, and relative abundance of iron (Fe) phases in PM2.5 samples from two locations in urban Los Angeles were investigated using a combination of bulk and spatially resolved, element-specific spectroscopy and microscopy methods. Synchrotron X-ray absorption spectroscopy (XAS) of bulk samples in situ (i.e., without extraction or digestion) was used to quantify the relative fractions of major Fe phases, which were corroborated by spatially resolved spectro-microscopy measurements. Ferrihydrite (amorphous Fe(III)-hydroxide) comprised the largest Fe fraction (34-52%), with hematite (α-Fe2O3; 13-23%) and magnetite (Fe3O4; 10-24%) identified as major crystalline oxide components. An Fe-bearing phyllosilicate fraction (16-23%) was fit best with a reference spectrum of a natural illite/smectite mineral, and metallic Fe(0) was a relatively small (2-6%) but easily identified component. Sizes, morphologies, oxidation state, and trace element compositions of Fe-bearing PM from electron microscopy, electron energy loss spectroscopy (EELS), and scanning transmission X-ray microscopy (STXM) revealed variable and heterogeneous mixtures of Fe species and phases, often associated with carbonaceous material with evidence of surface oxidation. Ferrihydrite (or related Fe(III) hydroxide phases) was ubiquitous in PM samples. It forms as an oxidation or surface alteration product of crystalline Fe phases, and also occurs as coatings or nanoparticles dispersed with other phases as a result of environmental dissolution and re-precipitation reactions. The prevalence of ferrihydrite (and adsorbed Fe(III) has likely been underestimated in studies of ambient PM because it is non-crystalline, non-magnetic, more soluble than crystalline phases, and found in complex mixtures. Review of potential sources of different particle types suggests that the majority of Fe-bearing PM from these urban sites originates from anthropogenic activities, primarily abrasion products from vehicle braking systems and engine emissions from combustion and/or wear. These variable mixtures have a high probability for electron transfer reactions between Fe, redox-active metals such as copper, and reactive carbon species such as quinones. Our findings suggest the need to assess biological responses of specific Fe-bearing phases both individually and in combination to unravel mechanisms of adverse health effects of particulate Fe.
中文翻译:

使用光谱显微镜方法分析洛杉矶市区颗粒物 (PM2.5) 中的铁形态
采用体相和空间分辨、元素特异性光谱和显微镜方法相结合的方法,对洛杉矶市区两个地点的 PM2.5 样品中铁 (Fe) 相的形态、氧化态和相对丰度进行了研究。原位(即未经提取或消化)大块样品的同步加速器 X 射线吸收光谱 (XAS) 用于量化主要 Fe 相的相对分数,并通过空间分辨光谱显微镜测量得到证实。水铁矿(无定形 Fe(III)-氢氧化物)包含最大的 Fe 成分 (34-52%),其中赤铁矿 (α-Fe2O3;13-23%) 和磁铁矿 (Fe3O4;10-24%) 被确定为主要的结晶氧化物成分。含铁页硅酸盐部分 (16-23%) 最适合天然伊利石/蒙皂石矿物的参考光谱,金属 Fe(0) 是相对较小 (2-6%) 但易于识别的成分。通过电子显微镜、电子能量损失光谱 (EELS) 和扫描透射 X 射线显微镜 (STXM) 分析含铁 PM 的尺寸、形态、氧化态和微量元素组成,揭示了铁物种和相的可变且不均匀的混合物,通常与具有表面氧化证据的碳质材料有关。水铁矿(或相关的 Fe(III) 氢氧化物相)在 PM 样品中普遍存在。它作为结晶铁相的氧化或表面改变产物形成,并且由于环境溶解和再沉淀反应而以与其他相分散的涂层或纳米颗粒的形式出现。 在环境 PM 的研究中,水铁矿(和吸附的 Fe(III))的存在率可能被低估,因为它是非结晶、非磁性、比结晶相更易溶解,并且存在于复杂的混合物中。回顾不同颗粒的潜在来源类型表明,这些城市场所的大部分含铁颗粒物源自人类活动,主要是车辆制动系统的磨损产物以及燃烧和/或磨损引起的发动机排放,这些可变混合物很可能在铁、我们的研究结果表明,需要单独和组合评估特定含铁相的生物反应,以揭示颗粒铁对健康不利影响的机制。
更新日期:2021-01-01
中文翻译:

使用光谱显微镜方法分析洛杉矶市区颗粒物 (PM2.5) 中的铁形态
采用体相和空间分辨、元素特异性光谱和显微镜方法相结合的方法,对洛杉矶市区两个地点的 PM2.5 样品中铁 (Fe) 相的形态、氧化态和相对丰度进行了研究。原位(即未经提取或消化)大块样品的同步加速器 X 射线吸收光谱 (XAS) 用于量化主要 Fe 相的相对分数,并通过空间分辨光谱显微镜测量得到证实。水铁矿(无定形 Fe(III)-氢氧化物)包含最大的 Fe 成分 (34-52%),其中赤铁矿 (α-Fe2O3;13-23%) 和磁铁矿 (Fe3O4;10-24%) 被确定为主要的结晶氧化物成分。含铁页硅酸盐部分 (16-23%) 最适合天然伊利石/蒙皂石矿物的参考光谱,金属 Fe(0) 是相对较小 (2-6%) 但易于识别的成分。通过电子显微镜、电子能量损失光谱 (EELS) 和扫描透射 X 射线显微镜 (STXM) 分析含铁 PM 的尺寸、形态、氧化态和微量元素组成,揭示了铁物种和相的可变且不均匀的混合物,通常与具有表面氧化证据的碳质材料有关。水铁矿(或相关的 Fe(III) 氢氧化物相)在 PM 样品中普遍存在。它作为结晶铁相的氧化或表面改变产物形成,并且由于环境溶解和再沉淀反应而以与其他相分散的涂层或纳米颗粒的形式出现。 在环境 PM 的研究中,水铁矿(和吸附的 Fe(III))的存在率可能被低估,因为它是非结晶、非磁性、比结晶相更易溶解,并且存在于复杂的混合物中。回顾不同颗粒的潜在来源类型表明,这些城市场所的大部分含铁颗粒物源自人类活动,主要是车辆制动系统的磨损产物以及燃烧和/或磨损引起的发动机排放,这些可变混合物很可能在铁、我们的研究结果表明,需要单独和组合评估特定含铁相的生物反应,以揭示颗粒铁对健康不利影响的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号