当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microkinetic model for pH- and potential-dependent oxygen evolution during water splitting on Fe-doped β-NiOOH
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-10-13 , DOI: 10.1039/d0ee02292f Ananth Govind Rajan 1, 2, 3, 4 , Emily A. Carter 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-10-13 , DOI: 10.1039/d0ee02292f Ananth Govind Rajan 1, 2, 3, 4 , Emily A. Carter 1, 2, 3, 4, 5
Affiliation
|
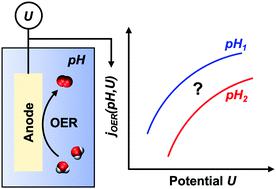
Electrochemical water splitting using excess renewable electricity is a CO2-free synthesis route for sustainable hydrogen production and a renewable-energy storage strategy. Electrolyte pH and electrode potential are key reactor operating conditions used to tune water-splitting kinetics whose influence remains incompletely understood. Here, we develop a microkinetic model, based on the Marcus theory of electron transfer, to predict the anodic oxygen-evolution-reaction (OER) current density (water-splitting rate) as a function of solution pH and electrode potential. Our model offers startling new insights into OER kinetics on Fe-doped β-nickel oxyhydroxide (β-NiOOH), a promising, inexpensive candidate for electrocatalyzing the OER, under alkaline solution conditions. Only four fitting parameters with clearly defined physical meaning – reorganization free energies (λ) for H+- and OH−-based reactions and work terms (w) for transporting H+/OH− ions and water from the bulk solution to the electrocatalyst surface – are required to reproduce experimental polarization curves (i.e., current-density vs. potential plots) at multiple alkaline pHs. The inclusion of work terms renders multiple steps rate-determining for the OER. The predicted λH+ = 0.744 eV is much smaller than λOH− = 2.474 eV, and wions = 0.607 eV is much larger than wwater = 0.065 eV, suggesting that the OER occurs primarily but not exclusively via water oxidation, rather than hydroxide oxidation, even under alkaline conditions. We show unequivocally that hydroxide oxidation also must occur as a minor channel, as accurate reproduction of polarization curves is impossible without it. We conclusively demonstrate the need to use the reversible, rather than the standard, hydrogen electrode as a reference in microkinetic models. Moreover, we deduce that the electrocatalyst surface is positively charged, contrary to inferences made in previous reports. Finally, we predict the following properties for the OER under high overpotential (≳0.3 V) conditions on Fe-doped β-NiOOH: a Tafel slope of ∼76 mV per decade, an effective charge transfer coefficient of 0.77, and an exchange current density of 3.5 μA cm−2 rivaling that of RuO2, one of the best noble-metal-containing water-splitting electrocatalysts. Our work substantially deepens the mechanistic understanding of water-splitting kinetics on inexpensive iron/nickel-oxyhydroxide-based electrocatalysts, which may help optimize operating conditions for their widespread deployment.
中文翻译:

Fe掺杂的β-NiOOH上水分解过程中pH依赖和电位依赖的氧气逸出的微动力学模型
使用过量可再生电力进行的电化学水分解是CO 2免于合成的路线,以实现可持续的制氢和可再生能源存储策略。电解质的pH值和电极电位是用于调节水分解动力学的关键反应器操作条件,其影响尚不完全清楚。在这里,我们基于马库斯电子转移理论建立了一个微动力学模型,以预测阳极氧演化反应(OER)电流密度(水分解速率)与溶液pH和电极电位的关系。我们的模型为掺铁的β-羟基氧化镍(β-NiOOH)的OER动力学提供了令人惊讶的新见解,这是一种在碱性溶液条件下用于电催化OER的有前途的廉价候选物。只有四个拟合参数具有明确定义的物理含义– H的重组自由能(λ)+ -和OH -基反应和工作条件(瓦特用于运输)H + / OH -从本体溶液到表面电离子和水-需要重现实验极化曲线(即,电流密度相对于电势曲线)在多个碱性pH值下。包含工作术语使OER可以确定多个步骤。预测λ ħ + = 0.744 eV的远小于λ OH - = 2.474 eV的,和瓦特离子= 0.607 eV的远大于瓦特水= 0.065 eV,这表明OER主要但并非仅通过即使在碱性条件下也可以用水氧化,而不是氢氧化物氧化。我们明确地表明,氢氧化物氧化也必须作为次要通道发生,因为没有它就不可能精确再现极化曲线。我们最终证明有必要使用可逆而不是标准的氢电极作为微动力学模型的参考。而且,我们推论出电催化剂表面带正电,这与以前的报道相反。最后,我们预测在高超电势(≳0.3V)条件下,掺铁的β-NiOOH上的OER具有以下特性:每十年的Tafel斜率为〜76 mV,有效电荷转移系数为0.77,交换电流密度3.5μAcm -2的强度可与RuO 2媲美,最好的含贵金属的水分解电催化剂之一。我们的工作极大地加深了对廉价的铁/羟基氧化镍基电催化剂的水分解动力学的机械理解,这可能有助于优化其广泛应用的操作条件。
更新日期:2020-11-12
中文翻译:

Fe掺杂的β-NiOOH上水分解过程中pH依赖和电位依赖的氧气逸出的微动力学模型
使用过量可再生电力进行的电化学水分解是CO 2免于合成的路线,以实现可持续的制氢和可再生能源存储策略。电解质的pH值和电极电位是用于调节水分解动力学的关键反应器操作条件,其影响尚不完全清楚。在这里,我们基于马库斯电子转移理论建立了一个微动力学模型,以预测阳极氧演化反应(OER)电流密度(水分解速率)与溶液pH和电极电位的关系。我们的模型为掺铁的β-羟基氧化镍(β-NiOOH)的OER动力学提供了令人惊讶的新见解,这是一种在碱性溶液条件下用于电催化OER的有前途的廉价候选物。只有四个拟合参数具有明确定义的物理含义– H的重组自由能(λ)+ -和OH -基反应和工作条件(瓦特用于运输)H + / OH -从本体溶液到表面电离子和水-需要重现实验极化曲线(即,电流密度相对于电势曲线)在多个碱性pH值下。包含工作术语使OER可以确定多个步骤。预测λ ħ + = 0.744 eV的远小于λ OH - = 2.474 eV的,和瓦特离子= 0.607 eV的远大于瓦特水= 0.065 eV,这表明OER主要但并非仅通过即使在碱性条件下也可以用水氧化,而不是氢氧化物氧化。我们明确地表明,氢氧化物氧化也必须作为次要通道发生,因为没有它就不可能精确再现极化曲线。我们最终证明有必要使用可逆而不是标准的氢电极作为微动力学模型的参考。而且,我们推论出电催化剂表面带正电,这与以前的报道相反。最后,我们预测在高超电势(≳0.3V)条件下,掺铁的β-NiOOH上的OER具有以下特性:每十年的Tafel斜率为〜76 mV,有效电荷转移系数为0.77,交换电流密度3.5μAcm -2的强度可与RuO 2媲美,最好的含贵金属的水分解电催化剂之一。我们的工作极大地加深了对廉价的铁/羟基氧化镍基电催化剂的水分解动力学的机械理解,这可能有助于优化其广泛应用的操作条件。










































 京公网安备 11010802027423号
京公网安备 11010802027423号