当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic understanding of ethane dehydrogenation and aromatization over Zn/ZSM-5: effects of Zn modification and CO2 co-reactant
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-10-13 , DOI: 10.1039/d0cy01566k Huahua Fan 1, 2, 3, 4, 5 , Xiaowa Nie 1, 2, 3, 4, 5 , Haozhi Wang 1, 2, 3, 4, 5 , Michael J. Janik 6, 7, 8, 9 , Chunshan Song 1, 2, 3, 4, 5 , Xinwen Guo 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-10-13 , DOI: 10.1039/d0cy01566k Huahua Fan 1, 2, 3, 4, 5 , Xiaowa Nie 1, 2, 3, 4, 5 , Haozhi Wang 1, 2, 3, 4, 5 , Michael J. Janik 6, 7, 8, 9 , Chunshan Song 1, 2, 3, 4, 5 , Xinwen Guo 1, 2, 3, 4, 5
Affiliation
|
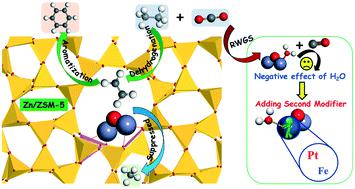
Due to the vigorous development of shale gas production technology, the aromatization of light alkanes has become more attractive for the chemical industry. Ethane dehydrogenation/aromatization over Zn/ZSM-5 catalyst was investigated using density functional theory calculations to clarify the intrinsic effects of introducing a Zn modifier and CO2 co-reactant on the catalytic activity and performance. Introducing Zn to HZSM-5 resulted in the creation of new active sites composed of (Zn–O–Zn)2+ species and thus altered the reaction pathways and reduced the kinetic barriers of ethane dehydrogenation. Moreover, Zn/ZSM-5 significantly suppressed methane by-product formation as compared to the unmodified ZSM-5, leading to an increased selectivity to aromatic products. In the presence of CO2, the H2O produced via the reverse water gas shift (RWGS) reaction could hydrolyze the (Zn–O–Zn)2+ active sites and produce weaker acid sites, which correspond to the increased barriers for ethane dehydrogenation. The participation of H2O in ethane conversion also reduced the catalytic activity of Zn/ZSM-5. The present DFT results predict that adding Pt or Fe as a second modifier for Zn/ZSM-5 helps to prevent the hydrolysis of (Zn–O–Zn)2+ active sites and minimize the negative effect of H2O on ethane conversion, potentially leading to CO2-assisted dehydrogenation/aromatization of light alkanes.
中文翻译:

Zn / ZSM-5上乙烷脱氢和芳构化的机理理解:Zn改性和CO2共反应物的影响
由于页岩气生产技术的蓬勃发展,轻链烷烃的芳构化已对化学工业更具吸引力。使用密度泛函理论计算研究了Zn / ZSM-5催化剂上的乙烷脱氢/芳构化,以阐明引入Zn改性剂和CO 2共反应剂对催化活性和性能的内在影响。在HZSM-5中引入Zn导致了由(Zn–O–Zn)2+物种组成的新活性位点的产生,从而改变了反应途径并减少了乙烷脱氢的动力学障碍。此外,与未改性的ZSM-5相比,Zn / ZSM-5显着抑制了甲烷副产物的形成,从而提高了对芳族产物的选择性。在CO存在下如图2所示,通过反向水煤气变换(RWGS)反应产生的H 2 O可以水解(Zn–O–Zn) 2+活性位点,并产生较弱的酸位点,这对应于乙烷脱氢的障碍增加。H 2 O参与乙烷转化也降低了Zn / ZSM-5的催化活性。目前的DFT结果表明,添加Pt或Fe作为Zn / ZSM-5的第二种改性剂有助于防止(Zn–O–Zn) 2+活性位点水解,并最大程度降低H 2 O对乙烷转化的负面影响,可能导致CO 2辅助的轻烷烃脱氢/芳构化。
更新日期:2020-11-03
中文翻译:

Zn / ZSM-5上乙烷脱氢和芳构化的机理理解:Zn改性和CO2共反应物的影响
由于页岩气生产技术的蓬勃发展,轻链烷烃的芳构化已对化学工业更具吸引力。使用密度泛函理论计算研究了Zn / ZSM-5催化剂上的乙烷脱氢/芳构化,以阐明引入Zn改性剂和CO 2共反应剂对催化活性和性能的内在影响。在HZSM-5中引入Zn导致了由(Zn–O–Zn)2+物种组成的新活性位点的产生,从而改变了反应途径并减少了乙烷脱氢的动力学障碍。此外,与未改性的ZSM-5相比,Zn / ZSM-5显着抑制了甲烷副产物的形成,从而提高了对芳族产物的选择性。在CO存在下如图2所示,通过反向水煤气变换(RWGS)反应产生的H 2 O可以水解(Zn–O–Zn) 2+活性位点,并产生较弱的酸位点,这对应于乙烷脱氢的障碍增加。H 2 O参与乙烷转化也降低了Zn / ZSM-5的催化活性。目前的DFT结果表明,添加Pt或Fe作为Zn / ZSM-5的第二种改性剂有助于防止(Zn–O–Zn) 2+活性位点水解,并最大程度降低H 2 O对乙烷转化的负面影响,可能导致CO 2辅助的轻烷烃脱氢/芳构化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号