当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-Metal-Free Cross-Coupling Reaction of Iodocarboranes with Terminal Alkynes Enabled by UV Light: Synthesis of 1-Alkynyl-o-Carboranes and Carborane-Fused Cyclics
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-13 , DOI: 10.1021/jacs.0c08652 Hangcheng Ni 1 , Zhenpin Lu 1 , Zuowei Xie 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-13 , DOI: 10.1021/jacs.0c08652 Hangcheng Ni 1 , Zhenpin Lu 1 , Zuowei Xie 1
Affiliation
|
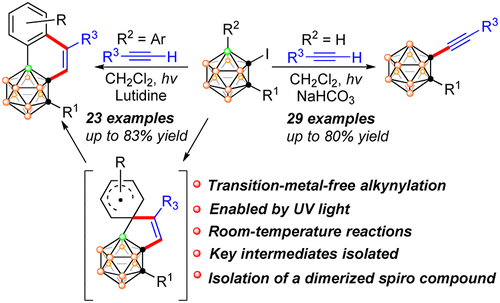
A transition-metal-free coupling protocol between iodocarboranes and terminal alkynes enabled by light at room temperature has been developed, leading to the synthesis of a variety of 1-alkynyl-o-carboranes. Moreover, following this strategy, the introduction of 1-I-3-aryl-o-carboranes or 1-I-2-aryl-o-carboranes results in the formation of o-carborane-fused cyclics. Interestingly, when 1-I-3-(p-R-C6H4)-o-carboranes are chosen as coupling partners, unexpected R-group migration products are also isolated. On the basis of the results of control experiments and isolation of the key intermediates, a possible reaction mechanism is then proposed, involving the formation of spiro radical species.
中文翻译:

紫外光使碘碳硼烷与末端炔烃的无过渡金属交叉偶联反应:1-炔基-邻-碳硼烷和碳硼烷稠环化合物的合成
已经开发了在室温下通过光实现碘碳硼烷和末端炔烃之间的无过渡金属偶联方案,从而合成了各种 1-炔基-o-碳硼烷。此外,按照该策略,1-I-3-芳基-o-碳硼烷或1-I-2-芳基-o-碳硼烷的引入导致形成邻-碳硼烷稠合环。Interestingly, when 1-I-3-(pR-C6H4)-o-carboranes are chosen as coupling partners, unexpected R-group migration products are also isolated. 在对照实验和关键中间体分离的结果的基础上,提出了一种可能的反应机理,涉及螺自由基物种的形成。
更新日期:2020-10-13
中文翻译:

紫外光使碘碳硼烷与末端炔烃的无过渡金属交叉偶联反应:1-炔基-邻-碳硼烷和碳硼烷稠环化合物的合成
已经开发了在室温下通过光实现碘碳硼烷和末端炔烃之间的无过渡金属偶联方案,从而合成了各种 1-炔基-o-碳硼烷。此外,按照该策略,1-I-3-芳基-o-碳硼烷或1-I-2-芳基-o-碳硼烷的引入导致形成邻-碳硼烷稠合环。Interestingly, when 1-I-3-(pR-C6H4)-o-carboranes are chosen as coupling partners, unexpected R-group migration products are also isolated. 在对照实验和关键中间体分离的结果的基础上,提出了一种可能的反应机理,涉及螺自由基物种的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号