当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
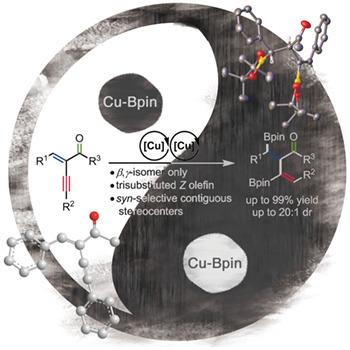
Access to Stereodefined Multifunctionalized β,γ‐Unsaturated Ketones via Chemo‐, Regio‐ and Diastereoselective Copper‐Catalyzed Diborylation of Cross‐Conjugated Enynones†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-10-12 , DOI: 10.1002/cjoc.202000475 Shuai Zhang 1 , Xinhua Duan 2, 3 , Pengfei Li 1, 3, 4
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-10-12 , DOI: 10.1002/cjoc.202000475 Shuai Zhang 1 , Xinhua Duan 2, 3 , Pengfei Li 1, 3, 4
Affiliation
|
Stereodefined β,γ‐unsaturated carbonyl compounds are important yet synthetically challenging units in natural products and drugs. Disclosed herein is a mild, rapid copper‐catalyzed diborylation reaction of cross‐conjugated enynones as a step‐economic and modular approach to stereodefined multifunctionalized β,γ‐unsaturated ketones by fine control of chemo‐, regio‐, Z/E and diastereoselectivity. The substrate scope was examined and a possible catalytic cycle was proposed to explain the multifaceted selectivity.
中文翻译:

通过交叉共轭烯酮的化学,区域和非对映选择性铜催化双官能化获得立体定义的多功能β,γ-不饱和酮
立体定义的β,γ-不饱和羰基化合物在天然产物和药物中是重要的但具有合成挑战性的单元。本文公开了一种交叉共轭烯酮的温和,快速的铜催化重氮基化反应,可通过精细控制化学,区域,Z / E和非对映选择性的步骤经济和模块化的方法来立体定义多官能化的β,γ-不饱和酮。检查了底物范围,并提出了可能的催化循环来解释多方面的选择性。
更新日期:2020-10-12

中文翻译:

通过交叉共轭烯酮的化学,区域和非对映选择性铜催化双官能化获得立体定义的多功能β,γ-不饱和酮
立体定义的β,γ-不饱和羰基化合物在天然产物和药物中是重要的但具有合成挑战性的单元。本文公开了一种交叉共轭烯酮的温和,快速的铜催化重氮基化反应,可通过精细控制化学,区域,Z / E和非对映选择性的步骤经济和模块化的方法来立体定义多官能化的β,γ-不饱和酮。检查了底物范围,并提出了可能的催化循环来解释多方面的选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号