当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tissue‐specific mesenchymal stem cell‐dependent osteogenesis in highly porous chitosan‐based bone analogs
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-10-13 , DOI: 10.1002/sctm.19-0385 Swati Midha 1 , Krishan G Jain 1 , Nitu Bhaskar 2 , Amtoj Kaur 1 , Sonali Rawat 1 , Shibashish Giri 3, 4 , Bikramjit Basu 2 , Sujata Mohanty 1
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-10-13 , DOI: 10.1002/sctm.19-0385 Swati Midha 1 , Krishan G Jain 1 , Nitu Bhaskar 2 , Amtoj Kaur 1 , Sonali Rawat 1 , Shibashish Giri 3, 4 , Bikramjit Basu 2 , Sujata Mohanty 1
Affiliation
|
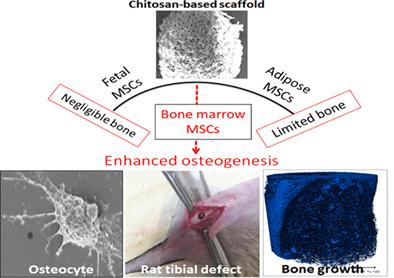
Among conventional fabrication techniques, freeze‐drying process has widely been investigated for polymeric implants. However, the understanding of the stem cell progenitor‐dependent cell functionality modulation and quantitative analysis of early osseointegration of highly porous scaffolds have not been explored. Here, we developed a novel, highly porous, multimaterial composite, chitosan/hydroxyapatite/polycaprolactone (CHT/HA/PCL). The in vitro studies have been performed using mesenchymal stem cells (MSCs) from three tissue sources: human bone marrow‐derived MSCs (BM‐MSCs), adipose‐derived MSCs (AD‐MSCs), and Wharton's jelly‐derived MSCs (WJ‐MSCs). Although cell attachment and metabolic activity [3‐4,5‐dimethylthiazol‐2yl‐(2,5 diphenyl‐2H‐tetrazoliumbromide) assay] were ore enhanced in WJ‐MSC‐laden CHT/HA/PCL composites, scanning electron microscopy, real‐time gene expression (alkaline phosphatase [ALP], collagen type I [Col I], osteocalcin [OCN], and bone morphogenetic protein 4 [BMP‐4]), and immunostaining (COL I, β‐CATENIN, OCN, and SCLEROSTIN [SOST]) demonstrated pronounced osteogenesis with terminal differentiation on BM‐MSC‐laden CHT/HA/PCL composites only. The enhanced cell functionality on CHT/HA/PCL composites was explained in terms of interplay among the surface properties and the optimal source of MSCs. In addition, osteogenesis in rat tibial model over 6 weeks confirmed a better ratio of bone volume to the total volume for BM‐MSC‐laden composites over scaffold‐only and defect‐only groups. The clinically conformant combination of 3D porous architecture with pore sizes varying in the range of 20 to 200 μm together with controlled in vitro degradation and early osseointegration establish the potential of CHT/HA/PCL composite as a potential cancellous bone analog.
中文翻译:

高度多孔壳聚糖基骨类似物中组织特异性间充质干细胞依赖性成骨
在传统的制造技术中,冷冻干燥工艺已被广泛研究用于聚合物植入物。然而,尚未探索对干细胞祖细胞依赖性细胞功能调节的理解和高度多孔支架早期骨整合的定量分析。在这里,我们开发了一种新型、高度多孔的多材料复合材料,壳聚糖/羟基磷灰石/聚己内酯 (CHT/HA/PCL)。体外研究使用来自三种组织来源的间充质干细胞 (MSCs):人骨髓来源的 MSCs (BM-MSCs)、脂肪来源的 MSCs (AD-MSCs) 和沃顿氏胶来源的 MSCs (WJ- MSC)。尽管载有 WJ-MSC 的 CHT/HA/PCL 复合材料中的细胞附着和代谢活性 [3-4,5-二甲基噻唑-2-(2,5-二苯基-2H-四唑溴化物) 测定法] 得到了增强,ALP ]、I 型胶原蛋白 [ Col I ]、骨钙素 [ OCN ] 和骨形态发生蛋白 4 [ BMP-4])和免疫染色(COL I、β-CATENIN、OCN 和 SCLEROSTIN [SOST])仅在载有 BM-MSC 的 CHT/HA/PCL 复合材料上显示出明显的成骨和终末分化。CHT/HA/PCL 复合材料上增强的细胞功能性被解释为表面性质和 MSCs 的最佳来源之间的相互作用。此外,6 周内大鼠胫骨模型中的成骨证实,与仅支架组和仅缺损组相比,载有 BM-MSC 的复合材料的骨体积与总体积的比例更好。3D 多孔结构与孔径在 20 到 200 μm 范围内变化的临床一致性组合以及受控的体外降解和早期骨整合确立了 CHT/HA/PCL 复合材料作为潜在松质骨类似物的潜力。
更新日期:2020-10-13
中文翻译:

高度多孔壳聚糖基骨类似物中组织特异性间充质干细胞依赖性成骨
在传统的制造技术中,冷冻干燥工艺已被广泛研究用于聚合物植入物。然而,尚未探索对干细胞祖细胞依赖性细胞功能调节的理解和高度多孔支架早期骨整合的定量分析。在这里,我们开发了一种新型、高度多孔的多材料复合材料,壳聚糖/羟基磷灰石/聚己内酯 (CHT/HA/PCL)。体外研究使用来自三种组织来源的间充质干细胞 (MSCs):人骨髓来源的 MSCs (BM-MSCs)、脂肪来源的 MSCs (AD-MSCs) 和沃顿氏胶来源的 MSCs (WJ- MSC)。尽管载有 WJ-MSC 的 CHT/HA/PCL 复合材料中的细胞附着和代谢活性 [3-4,5-二甲基噻唑-2-(2,5-二苯基-2H-四唑溴化物) 测定法] 得到了增强,ALP ]、I 型胶原蛋白 [ Col I ]、骨钙素 [ OCN ] 和骨形态发生蛋白 4 [ BMP-4])和免疫染色(COL I、β-CATENIN、OCN 和 SCLEROSTIN [SOST])仅在载有 BM-MSC 的 CHT/HA/PCL 复合材料上显示出明显的成骨和终末分化。CHT/HA/PCL 复合材料上增强的细胞功能性被解释为表面性质和 MSCs 的最佳来源之间的相互作用。此外,6 周内大鼠胫骨模型中的成骨证实,与仅支架组和仅缺损组相比,载有 BM-MSC 的复合材料的骨体积与总体积的比例更好。3D 多孔结构与孔径在 20 到 200 μm 范围内变化的临床一致性组合以及受控的体外降解和早期骨整合确立了 CHT/HA/PCL 复合材料作为潜在松质骨类似物的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号