当前位置:
X-MOL 学术
›
Surf. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of CeO2 nanotubes through different conditions of hydrothermal synthesis
Surfaces and Interfaces ( IF 5.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.surfin.2020.100746 A.P.B. dos Santos , T.C.M. Dantas , J.A.P. Costa , L.D. Souza , J.M. Soares , V.P.S. Caldeira , A.S. Araújo , A.G.D. Santos
Surfaces and Interfaces ( IF 5.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.surfin.2020.100746 A.P.B. dos Santos , T.C.M. Dantas , J.A.P. Costa , L.D. Souza , J.M. Soares , V.P.S. Caldeira , A.S. Araújo , A.G.D. Santos
|
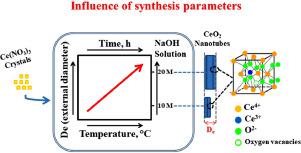
Abstract The synthesis control in obtaining CeO2 nanostructures is of fundamental importance for obtaining materials with desired properties. However, progress in the synthesis and control of the properties of these unidirectional (1D) nanomaterials remains a major challenge. In this work, alkaline hydrothermal synthesis without the use of a template was performed, under different reaction conditions (NaOH concentration, temperature and time of synthesis), and the influence on the morphological, structural and optical properties of CeO2 nanotubes (CeNTs) formed was discussed. In some cases, hydrated CeNTs, organized in a cubic fluorite structure, were obtained, with varying sizes, and varying quantity of oxygen vacancies, according to the condition of syntheses used, thus generating materials with different properties. It was observed that the external diameter (De) of the obtained nanotubes and oxygen vacancies increased the higher the NaOH concentration, the temperature and the time used in the reaction. Only the synthesis conditions of 5 M NaOH and 150°C do not promote the morphology of interest, with the formation of prisms and a mixture of nanotubes/nanocubes. The best synthesis conditions were obtained when using an alkaline concentration of 10 mol.L−1 at 125 °C for a reaction time of 72 h, since, under these conditions, a higher yield of nanostructures was obtained over an interesting size range (CeNTs with internal and external diameters of 7 and 24,6 nm, respectively). The band gap value of 2.60 eV, which is possibly responsible for the red shifting of band gap, indicates that these materials are photosensitive in the visible region. The mechanism involves the process of cerium salt dissolution and recrystallization of CeO2 nanotubes. This work shows, for the first time, that control of the formation, size and different quantities of Ce4+/Ce3+ of the CeNTs that can be reached by adjusting the synthesis parameters. Control in obtaining these nanostructures, combined with a narrow gap energy, it may show a differential performance for applications in the photocatalysis, sensors, nanomedicine and advanced electronic devices areas.
中文翻译:

通过不同的水热合成条件形成 CeO2 纳米管
摘要 获得 CeO2 纳米结构的合成控制对于获得具有所需性能的材料至关重要。然而,在合成和控制这些单向(1D)纳米材料的特性方面取得进展仍然是一个重大挑战。在这项工作中,在不使用模板的情况下进行碱性水热合成,在不同的反应条件(NaOH 浓度、合成温度和时间)下,对形成的 CeO2 纳米管 (CeNTs) 的形态、结构和光学性质的影响进行了研究。讨论。在某些情况下,根据所使用的合成条件,获得了以立方萤石结构组织的水合 CeNT,具有不同的尺寸和不同数量的氧空位,从而产生具有不同性质的材料。观察到所得纳米管的外径 (De) 和氧空位随着 NaOH 浓度、温度和反应时间的增加而增加。只有 5 M NaOH 和 150°C 的合成条件不会促进感兴趣的形态,形成棱柱和纳米管/纳米立方体的混合物。当在 125 °C 下使用 10 mol.L-1 的碱浓度,反应时间为 72 小时时,可以获得最佳合成条件,因为在这些条件下,在感兴趣的尺寸范围内获得了更高的纳米结构产量(CeNTs内径和外径分别为 7 和 24,6 nm)。2.60 eV 的带隙值可能是带隙红移的原因,表明这些材料在可见光区具有光敏性。其机理涉及CeO2纳米管的铈盐溶解和重结晶过程。这项工作首次表明,可以通过调整合成参数来控制 CeNTs 的形成、大小和不同数量的 Ce4+/Ce3+。控制获得这些纳米结构,结合窄间隙能量,它可能在光催化、传感器、纳米医学和先进电子设备领域的应用中表现出不同的性能。
更新日期:2020-12-01
中文翻译:

通过不同的水热合成条件形成 CeO2 纳米管
摘要 获得 CeO2 纳米结构的合成控制对于获得具有所需性能的材料至关重要。然而,在合成和控制这些单向(1D)纳米材料的特性方面取得进展仍然是一个重大挑战。在这项工作中,在不使用模板的情况下进行碱性水热合成,在不同的反应条件(NaOH 浓度、合成温度和时间)下,对形成的 CeO2 纳米管 (CeNTs) 的形态、结构和光学性质的影响进行了研究。讨论。在某些情况下,根据所使用的合成条件,获得了以立方萤石结构组织的水合 CeNT,具有不同的尺寸和不同数量的氧空位,从而产生具有不同性质的材料。观察到所得纳米管的外径 (De) 和氧空位随着 NaOH 浓度、温度和反应时间的增加而增加。只有 5 M NaOH 和 150°C 的合成条件不会促进感兴趣的形态,形成棱柱和纳米管/纳米立方体的混合物。当在 125 °C 下使用 10 mol.L-1 的碱浓度,反应时间为 72 小时时,可以获得最佳合成条件,因为在这些条件下,在感兴趣的尺寸范围内获得了更高的纳米结构产量(CeNTs内径和外径分别为 7 和 24,6 nm)。2.60 eV 的带隙值可能是带隙红移的原因,表明这些材料在可见光区具有光敏性。其机理涉及CeO2纳米管的铈盐溶解和重结晶过程。这项工作首次表明,可以通过调整合成参数来控制 CeNTs 的形成、大小和不同数量的 Ce4+/Ce3+。控制获得这些纳米结构,结合窄间隙能量,它可能在光催化、传感器、纳米医学和先进电子设备领域的应用中表现出不同的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号