当前位置:
X-MOL 学术
›
Opt. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoluminescence of Tb3+-doped LiF–BaF2–Lu2O3–Al2O3–SiO2 glasses
Optical Materials ( IF 3.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.optmat.2020.110513 Chenggang Zuo , Ligang Zhu , Zhihua Zhou , Anguo Xiao , Shaoyou Liu , Lin Li , Li Wan , Liying Zheng
Optical Materials ( IF 3.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.optmat.2020.110513 Chenggang Zuo , Ligang Zhu , Zhihua Zhou , Anguo Xiao , Shaoyou Liu , Lin Li , Li Wan , Liying Zheng
|
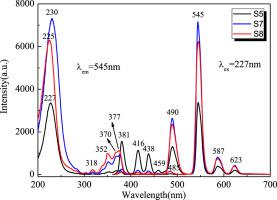
Abstract LiF–BaF2–Lu2O3–Al2O3–SiO2 glasses doped with Tb3+ ions were obtained by melt–quenching technique, and their transmission and photoluminescence properties were investigated. The X-ray diffraction (XRD) and luminescence decay profiles of these prepared samples have been obtained to confirm their structure and luminescence dynamics. The prepared oxyfluoride glasses doped with Tb3+ ions exhibit high transmittance in the visible light region, and the UV absorption edge appears red-shifted as the Tb2O3 content increases in the LiF–BaF2–Lu2O3–Al2O3–SiO2 oxyfluoride glasses. Under UV excitation, the Tb3+ ions emissions corresponding to 5D3 (5D4) → 7FJ (J = 6–3) electron transitions can be detected. With the increase of Tb2O3 content, the green light emission intensities caused by the Tb3+ ions 5D4 → 7FJ (J = 6–3) electron transitions are enhanced, while the blue light emission intensities induced by the Tb3+ ions 5D3 → 7FJ (J = 6–3) electron transitions first increased to the maximum until the introduction of Tb2O3 was equal to 0.3 mol, and then decreased with the Tb2O3 content further increase. The mechanism involved in the resonance energy transfer (RET) process between Tb3+ – Tb3+ ions was explained with an energy level diagram. And the luminescence decay times of Tb3+ ions electron transition originated from 5D4 energy level continue to decrease from 3.171 to 2.250 ms with increasing Tb2O3 content in the LiF–BaF2–Lu2O3–Al2O3–SiO2 glasses.
中文翻译:

Tb3+ 掺杂的 LiF-BaF2-Lu2O3-Al2O3-SiO2 玻璃的光致发光
摘要 采用熔融淬火技术制备了掺杂 Tb3+ 离子的 LiF-BaF2-Lu2O3-Al2O3-SiO2 玻璃,并研究了它们的透射和光致发光性能。已获得这些制备样品的 X 射线衍射 (XRD) 和发光衰减曲线,以确认它们的结构和发光动力学。制备的掺有 Tb3+ 离子的氟氧化物玻璃在可见光区域表现出高透射率,并且随着 LiF–BaF2–Lu2O3–Al2O3–SiO2 氟氧化物玻璃中 Tb2O3 含量的增加,紫外吸收边缘出现红移。在紫外激发下,可以检测到对应于 5D3 (5D4) → 7FJ (J = 6–3) 电子跃迁的 Tb3+ 离子发射。随着Tb2O3含量的增加,由Tb3+离子5D4→7FJ(J=6-3)电子跃迁引起的绿光发射强度增强,而由 Tb3+ 离子 5D3 → 7FJ (J = 6-3) 电子跃迁引起的蓝光发射强度首先增加到最大值,直到引入的 Tb2O3 等于 0.3 mol,然后随着 Tb2O3 含量的进一步增加而降低。Tb3+ – Tb3+ 离子之间共振能量转移 (RET) 过程中涉及的机制用能级图进行了解释。随着 LiF–BaF2–Lu2O3–Al2O3–SiO2 玻璃中 Tb2O3 含量的增加,源自 5D4 能级的 Tb3+ 离子电子跃迁的发光衰减时间从 3.171 ms 持续减少到 2.250 ms。Tb3+ – Tb3+ 离子之间共振能量转移 (RET) 过程中涉及的机制用能级图进行了解释。随着 LiF–BaF2–Lu2O3–Al2O3–SiO2 玻璃中 Tb2O3 含量的增加,源自 5D4 能级的 Tb3+ 离子电子跃迁的发光衰减时间从 3.171 ms 持续减少到 2.250 ms。Tb3+ – Tb3+ 离子之间共振能量转移 (RET) 过程中涉及的机制用能级图进行了解释。随着 LiF–BaF2–Lu2O3–Al2O3–SiO2 玻璃中 Tb2O3 含量的增加,源自 5D4 能级的 Tb3+ 离子电子跃迁的发光衰减时间从 3.171 ms 持续减少到 2.250 ms。
更新日期:2020-12-01
中文翻译:

Tb3+ 掺杂的 LiF-BaF2-Lu2O3-Al2O3-SiO2 玻璃的光致发光
摘要 采用熔融淬火技术制备了掺杂 Tb3+ 离子的 LiF-BaF2-Lu2O3-Al2O3-SiO2 玻璃,并研究了它们的透射和光致发光性能。已获得这些制备样品的 X 射线衍射 (XRD) 和发光衰减曲线,以确认它们的结构和发光动力学。制备的掺有 Tb3+ 离子的氟氧化物玻璃在可见光区域表现出高透射率,并且随着 LiF–BaF2–Lu2O3–Al2O3–SiO2 氟氧化物玻璃中 Tb2O3 含量的增加,紫外吸收边缘出现红移。在紫外激发下,可以检测到对应于 5D3 (5D4) → 7FJ (J = 6–3) 电子跃迁的 Tb3+ 离子发射。随着Tb2O3含量的增加,由Tb3+离子5D4→7FJ(J=6-3)电子跃迁引起的绿光发射强度增强,而由 Tb3+ 离子 5D3 → 7FJ (J = 6-3) 电子跃迁引起的蓝光发射强度首先增加到最大值,直到引入的 Tb2O3 等于 0.3 mol,然后随着 Tb2O3 含量的进一步增加而降低。Tb3+ – Tb3+ 离子之间共振能量转移 (RET) 过程中涉及的机制用能级图进行了解释。随着 LiF–BaF2–Lu2O3–Al2O3–SiO2 玻璃中 Tb2O3 含量的增加,源自 5D4 能级的 Tb3+ 离子电子跃迁的发光衰减时间从 3.171 ms 持续减少到 2.250 ms。Tb3+ – Tb3+ 离子之间共振能量转移 (RET) 过程中涉及的机制用能级图进行了解释。随着 LiF–BaF2–Lu2O3–Al2O3–SiO2 玻璃中 Tb2O3 含量的增加,源自 5D4 能级的 Tb3+ 离子电子跃迁的发光衰减时间从 3.171 ms 持续减少到 2.250 ms。Tb3+ – Tb3+ 离子之间共振能量转移 (RET) 过程中涉及的机制用能级图进行了解释。随着 LiF–BaF2–Lu2O3–Al2O3–SiO2 玻璃中 Tb2O3 含量的增加,源自 5D4 能级的 Tb3+ 离子电子跃迁的发光衰减时间从 3.171 ms 持续减少到 2.250 ms。











































 京公网安备 11010802027423号
京公网安备 11010802027423号