International Immunopharmacology ( IF 4.8 ) Pub Date : 2020-10-13 , DOI: 10.1016/j.intimp.2020.107102 Reza Malekzadeh 1 , Atefeh Abedini 2 , Behzad Mohsenpour 3 , Ehsan Sharifipour 4 , Roya Ghasemian 5 , Seyed Ali Javad-Mousavi 6 , Rozita Khodashahi 7 , Mahboobeh Darban 8 , Saeed Kalantari 9 , Nafiseh Abdollahi 10 , Mohammad Reza Salehi 11 , Abbas Rezaei Hosseinabadi 12 , Farzin Khorvash 13 , Melika Valizadeh 14 , Farzaneh Dastan 15 , Sahar Yousefian 15 , Hamed Hosseini 16 , Nassim Anjidani 17 , Payam Tabarsi 14
|
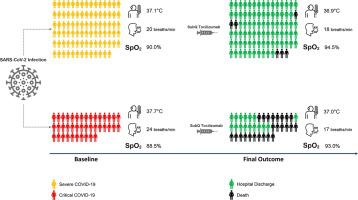
Potential therapeutic approaches in coronavirus disease 2019 (COVID-19) comprise antiviral and immunomodulatory agents; however, no immunomodulator drug has been approved. This multicenter, prospective, open-label, uncontrolled study aimed to assess the use of subcutaneous tocilizumab in adult patients with severe and critical COVID-19. Tocilizumab was added to the standard care of therapy at a dose of 324 mg (<100 kg bodyweight) or 486 mg (≥100 kg bodyweight). The study endpoints were all-cause mortality rate, changes in oxygen-support level, oxygen saturation, body temperature, respiratory rate, and laboratory variables during the study, and drug safety. Of 126 patients enrolled, 86 had severe and 40 had critical disease. Most patients were male (63.49%) and aged below 65 (78.57%). By day 14 of the study, 4.65% (4/86) of severe patients and 50.00% (20/40) of critical patients died. By the end, 6.98% (6/86) of severe patients and 60.00% (24/40) of critical patients died. Outcomes concerning three additional endpoints (oral temperature, oxygen saturation, and respiratory rate) were significantly improved as early as three days after tocilizumab administration in both groups of subjects, more considerably in severe patients. Significant improvement in the required level of oxygenation was reported in severe patients seven days after tocilizumab administration. No tocilizumab-related serious adverse event occurred in this study. Subcutaneous tocilizumab might improve some clinical parameters and reduce the risk of death in COVID-19 patients, particularly if used in the early stages of respiratory failure.
中文翻译:

皮下注射托珠单抗治疗重症和危重症 COVID-19 成人:一项前瞻性开放标签非对照多中心试验
2019 年冠状病毒病 (COVID-19) 的潜在治疗方法包括抗病毒药物和免疫调节药物;然而,尚未批准任何免疫调节药物。这项多中心、前瞻性、开放标签、非对照研究旨在评估皮下注射托珠单抗在重症和危重症 COVID-19 成人患者中的使用情况。托珠单抗以 324 mg(<100 kg 体重)或 486 mg(≥100 kg 体重)的剂量添加到标准治疗护理中。研究终点是全因死亡率、研究期间氧支持水平、氧饱和度、体温、呼吸频率和实验室变量的变化以及药物安全性。入组的 126 名患者中,86 名重症患者,40 名重症患者。大多数患者为男性(63.49%),年龄在65岁以下(78.57%)。截至研究第14天,4.65%(4/86)的重症患者和50.00%(20/40)的重症患者死亡。截至年底,重症患者死亡率为6.98%(6/86),重症患者死亡率为60.00%(24/40)。两组受试者在托珠单抗给药三天后,有关另外三个终点(口腔温度、氧饱和度和呼吸频率)的结果均显着改善,重症患者的改善更为显着。据报道,重症患者在托珠单抗给药 7 天后,所需的氧合水平显着改善。本研究中未发生与托珠单抗相关的严重不良事件。皮下注射托珠单抗可能会改善 COVID-19 患者的一些临床参数并降低死亡风险,特别是在呼吸衰竭的早期阶段使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号