当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Equilibria for the Quaternary System: Diisopropyl Ether + n-Pentanol + Phenol + Water at 298.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-10-12 , DOI: 10.1021/acs.jced.0c00354 Yangyang Wu 1 , Bokun Chen 1 , Siyu Yang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-10-12 , DOI: 10.1021/acs.jced.0c00354 Yangyang Wu 1 , Bokun Chen 1 , Siyu Yang 1
Affiliation
|
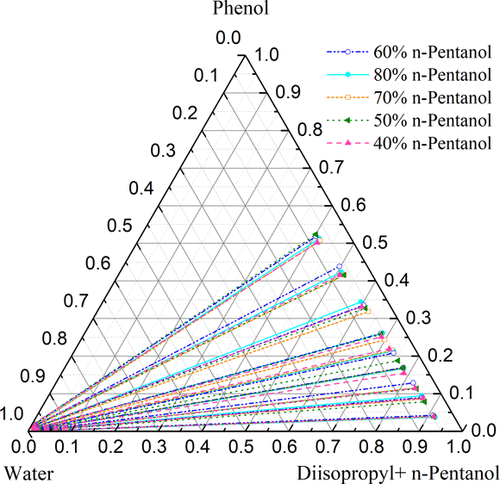
The synergistic extraction of phenol from aqueous solution with diisopropyl ether (DIPE) and n-pentanol was studied at 298.15 K under 101.3 kPa. The liquid–liquid equilibrium (LLE) data of the quaternary system (DIPE + n-pentanol + phenol + water) were studied using several proportions of mixed solvents, and the data of the ternary system (DIPE + phenol + water) were supplemented. Compared with single-solvent DIPE or n-pentanol, the extraction effect of the mixture (DIPE + n-pentanol) on phenol was significantly improved. The extraction efficiency was evaluated by calculating the distribution (D) and separation coefficient (S). Furthermore, the experimental LLE data were regressed with NRTL and UNIQUAC thermodynamic models to obtain the corresponding binary interaction parameters. The difference between the calculated value and the experimental value is less than 2%, calculated by relative root mean square deviation, indicating that the calculated and experimental results are in good agreement.
中文翻译:

四元体系的液-液平衡:298.15 K的二异丙醚+正戊醇+苯酚+水
在298.15 K和101.3 kPa下研究了用二异丙醚(DIPE)和正戊醇从水溶液中协同萃取苯酚的过程。使用几种比例的混合溶剂研究了四元体系(DIPE +正戊醇+苯酚+水)的液-液平衡(LLE)数据,并补充了三元体系(DIPE +苯酚+水)的数据。与单溶剂DIPE或正戊醇相比,混合物(DIPE +正戊醇)对苯酚的萃取效果得到了显着改善。通过计算分布(D)和分离系数(S)。此外,用NRTL和UNIQUAC热力学模型对LLE实验数据进行回归,得到相应的二元相互作用参数。通过相对均方根偏差计算得出的计算值与实验值之差小于2%,表明计算结果与实验结果吻合良好。
更新日期:2020-11-12
中文翻译:

四元体系的液-液平衡:298.15 K的二异丙醚+正戊醇+苯酚+水
在298.15 K和101.3 kPa下研究了用二异丙醚(DIPE)和正戊醇从水溶液中协同萃取苯酚的过程。使用几种比例的混合溶剂研究了四元体系(DIPE +正戊醇+苯酚+水)的液-液平衡(LLE)数据,并补充了三元体系(DIPE +苯酚+水)的数据。与单溶剂DIPE或正戊醇相比,混合物(DIPE +正戊醇)对苯酚的萃取效果得到了显着改善。通过计算分布(D)和分离系数(S)。此外,用NRTL和UNIQUAC热力学模型对LLE实验数据进行回归,得到相应的二元相互作用参数。通过相对均方根偏差计算得出的计算值与实验值之差小于2%,表明计算结果与实验结果吻合良好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号