当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Key Glycine in Bacterial Steroid-Degrading Acyl-CoA Dehydrogenases Allows Flavin-Ring Repositioning and Modulates Substrate Side Chain Specificity
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-11 , DOI: 10.1021/acs.biochem.0c00568 Alexander J. Stirling 1 , Stephanie E. Gilbert 1 , Megan Conner 1 , Evan Mallette 1 , Matthew S. Kimber 1 , Stephen Y. K. Seah 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-11 , DOI: 10.1021/acs.biochem.0c00568 Alexander J. Stirling 1 , Stephanie E. Gilbert 1 , Megan Conner 1 , Evan Mallette 1 , Matthew S. Kimber 1 , Stephen Y. K. Seah 1
Affiliation
|
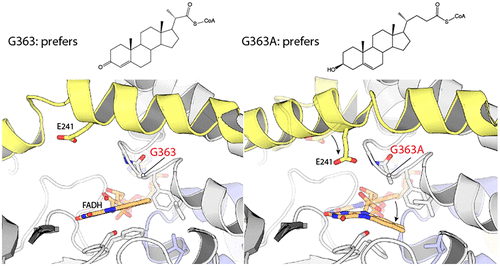
A wide variety of steroid metabolites synthesized by eukaryotes are all ultimately catabolized by bacteria; while generally saprophytic, pathogenic Mycobacteria have repurposed these pathways to utilize host intracellular cholesterol pools. Steroid degradation is complex, but a recurring theme is that cycles of β-oxidation are used to iteratively remove acetyl- or propanoyl-CoA groups. These β-oxidation cycles are initiated by the FAD-dependent oxidation of acyl groups, catalyzed by acyl-CoA dehydrogenases (ACADs). We show here that the tcur3481 and tcur3483 genes of Thermomonospora curvata encode subunits of a single ACAD that degrades steroid side chains with a preference for three-carbon over five-carbon substituents. The structure confirms that this enzyme is heterotetrameric, with active sites only in the Tcur3483 subunits. In comparison with the steroid ACAD FadE26-FadE27 from Mycobacterium tuberculosis, the active site is narrower and closed at the steroid-binding end, suggesting that Tcur3481–Tcur3483 is in a catalytically productive state, while FadE26-FadE27 is opened up to allow substrate entry. The flavin rings in Tcur3481-Tcur3483 sit in an unusual pocket created by Gly363, a residue conserved as Ala in steroid ACADs narrowly specific for five-carbon side chains, including FadE34. A Gly363Ala variant of Tcur3481-Tcur3483 prefers five-carbon side chains, while an inverse Ala691Gly FadE34 variant enables three-carbon side chain steroid oxidation. We determined the structure of the Tcur3483 Gly363Ala variant, showing that the flavin rings shift into the more conventional position. Modeling suggests that the shifted flavin position made possible by Gly363 is required to allow the bulky, inflexible three-carbon steroid to bind productively in the active site.
中文翻译:

细菌类固醇降解酰基辅酶A脱氢酶中的关键甘氨酸允许黄素环重新定位并调节底物侧链特异性。
真核生物合成的多种类固醇代谢物最终都被细菌分解代谢。尽管通常腐生,致病的分枝杆菌已将这些途径重新利用为利用宿主细胞内胆固醇库。类固醇的降解是复杂的,但是一个反复出现的主题是,使用β-氧化循环来迭代地去除乙酰基或丙酰基-CoA基团。这些β-氧化循环是由酰基辅酶A脱氢酶(ACAD)催化的FAD依赖性酰基氧化引发的。我们在这里显示了Thermomonospora curvata的tcur3481和tcur3483基因编码单个ACAD的亚基,可降解类固醇侧链,优先选择三碳取代基,而不是五碳取代基。结构证实该酶是异四聚体,仅在Tcur3483亚基中具有活性位点。与来自结核分枝杆菌的类固醇ACAD FadE26-FadE27相比,活性位点更窄,在类固醇结合端封闭,表明Tcur3481-Tcur3483处于催化生产状态,而FadE26-FadE27打开以允许底物进入。Tcur3481-Tcur3483中的黄素环位于一个不寻常的口袋中,该口袋由Gly363创建,Gly363是类固醇ACAD中的一个残基,保守于Ala,该残基专门针对五碳侧链,包括FadE34。Tcur3481-Tcur3483的Gly363Ala变异体偏爱五碳侧链,而反向Ala691Gly FadE34变异体则能使三碳侧链甾体氧化。我们确定了Tcur3483 Gly363Ala变体的结构,表明黄素环转移到了更常规的位置。建模表明,需要使用Gly363转移的黄素位置才能使体积大,
更新日期:2020-10-28
中文翻译:

细菌类固醇降解酰基辅酶A脱氢酶中的关键甘氨酸允许黄素环重新定位并调节底物侧链特异性。
真核生物合成的多种类固醇代谢物最终都被细菌分解代谢。尽管通常腐生,致病的分枝杆菌已将这些途径重新利用为利用宿主细胞内胆固醇库。类固醇的降解是复杂的,但是一个反复出现的主题是,使用β-氧化循环来迭代地去除乙酰基或丙酰基-CoA基团。这些β-氧化循环是由酰基辅酶A脱氢酶(ACAD)催化的FAD依赖性酰基氧化引发的。我们在这里显示了Thermomonospora curvata的tcur3481和tcur3483基因编码单个ACAD的亚基,可降解类固醇侧链,优先选择三碳取代基,而不是五碳取代基。结构证实该酶是异四聚体,仅在Tcur3483亚基中具有活性位点。与来自结核分枝杆菌的类固醇ACAD FadE26-FadE27相比,活性位点更窄,在类固醇结合端封闭,表明Tcur3481-Tcur3483处于催化生产状态,而FadE26-FadE27打开以允许底物进入。Tcur3481-Tcur3483中的黄素环位于一个不寻常的口袋中,该口袋由Gly363创建,Gly363是类固醇ACAD中的一个残基,保守于Ala,该残基专门针对五碳侧链,包括FadE34。Tcur3481-Tcur3483的Gly363Ala变异体偏爱五碳侧链,而反向Ala691Gly FadE34变异体则能使三碳侧链甾体氧化。我们确定了Tcur3483 Gly363Ala变体的结构,表明黄素环转移到了更常规的位置。建模表明,需要使用Gly363转移的黄素位置才能使体积大,











































 京公网安备 11010802027423号
京公网安备 11010802027423号