当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SAXS reveals highly flexible interdomain linkers of tandem acyl carrier protein–thioesterase domains from a fungal non‐reducing polyketide synthase
FEBS Letters ( IF 3.0 ) Pub Date : 2020-10-26 , DOI: 10.1002/1873-3468.13954 Waraporn Bunnak 1 , Ashley J Winter 2 , Colin M Lazarus 3 , Matthew P Crump 2 , Paul R Race 4, 5 , Pakorn Wattana-Amorn 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-10-26 , DOI: 10.1002/1873-3468.13954 Waraporn Bunnak 1 , Ashley J Winter 2 , Colin M Lazarus 3 , Matthew P Crump 2 , Paul R Race 4, 5 , Pakorn Wattana-Amorn 1
Affiliation
|
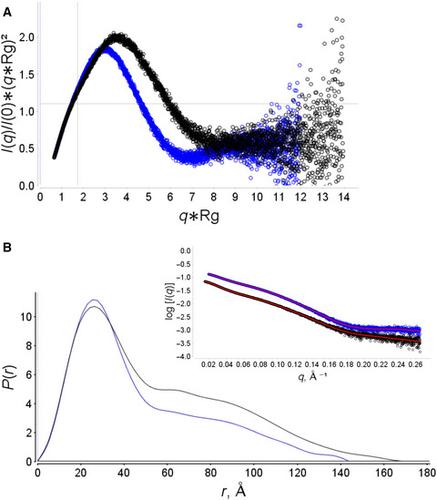
Menisporopsin A is a fungal bioactive macrocyclic polylactone, the biosynthesis of which requires only reducing (R) and nonreducing (NR) polyketide synthases (PKSs) to guide a series of esterification and cyclolactonization reactions. There is no structural information pertaining to these PKSs. Here, we report the solution characterization of singlet and doublet acyl carrier protein (ACP2 and ACP1‐ACP2)–thioesterase (TE) domains from NR‐PKS involved in menisporopsin A biosynthesis. Small‐angle X‐ray scattering (SAXS) studies in combination with homology modelling reveal that these polypeptides adopt a distinctive beads‐on‐a‐string configuration, characterized by the presence of highly flexible interdomain linkers. These models provide a platform for studying domain organization and interdomain interactions in fungal NR‐PKSs, which may be of value in directing the design of functionally optimized polyketide scaffolds.
中文翻译:

SAXS 揭示了来自真菌非还原性聚酮化合物合酶的串联酰基载体蛋白-硫酯酶结构域的高度灵活的域间接头
Menisporopsin A 是一种真菌生物活性大环聚内酯,其生物合成只需要还原 (R) 和非还原 (NR) 聚酮合酶 (PKS) 来指导一系列酯化和环内酯化反应。没有与这些 PKS 相关的结构信息。在这里,我们报告了参与半孢菌素 A 生物合成的来自 NR-PKS 的单线态和双线态酰基载体蛋白(ACP2 和 ACP1-ACP2)-硫酯酶(TE)结构域的溶液表征。小角度 X 射线散射 (SAXS) 研究与同源性建模相结合,表明这些多肽采用独特的串珠结构,其特征是存在高度灵活的域间连接子。这些模型为研究真菌 NR-PKS 中的域组织和域间相互作用提供了平台,
更新日期:2020-10-26
中文翻译:

SAXS 揭示了来自真菌非还原性聚酮化合物合酶的串联酰基载体蛋白-硫酯酶结构域的高度灵活的域间接头
Menisporopsin A 是一种真菌生物活性大环聚内酯,其生物合成只需要还原 (R) 和非还原 (NR) 聚酮合酶 (PKS) 来指导一系列酯化和环内酯化反应。没有与这些 PKS 相关的结构信息。在这里,我们报告了参与半孢菌素 A 生物合成的来自 NR-PKS 的单线态和双线态酰基载体蛋白(ACP2 和 ACP1-ACP2)-硫酯酶(TE)结构域的溶液表征。小角度 X 射线散射 (SAXS) 研究与同源性建模相结合,表明这些多肽采用独特的串珠结构,其特征是存在高度灵活的域间连接子。这些模型为研究真菌 NR-PKS 中的域组织和域间相互作用提供了平台,











































 京公网安备 11010802027423号
京公网安备 11010802027423号