当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent Advances in the Synthesis of C2‐Functionalized Pyridines and Quinolines Using N‐Oxide Chemistry
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-11 , DOI: 10.1002/adsc.202000910 Dong Wang 1, 2 , Laurent Désaubry 2, 3 , Gaoyu Li 2 , Mindong Huang 2 , Shixin Zheng 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-11 , DOI: 10.1002/adsc.202000910 Dong Wang 1, 2 , Laurent Désaubry 2, 3 , Gaoyu Li 2 , Mindong Huang 2 , Shixin Zheng 2
Affiliation
|
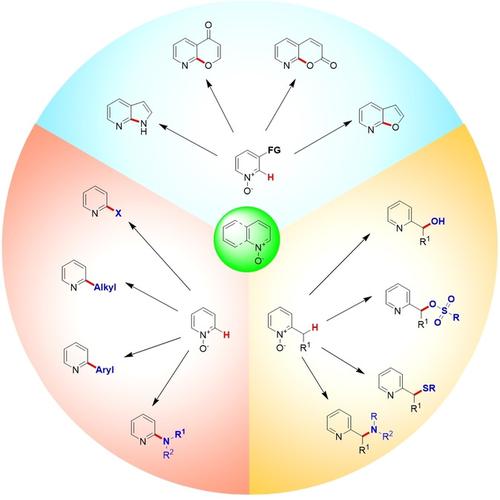
While remarkable progress has recently been made for the direct C−H‐functionalization of azines, its application is still limited by a lack of accessible functional groups (primarily carbon‐based) and poor regioselectivity. In contrast, C2‐functionalized pyridines and quinolines can be easily synthesized by treating readily available N‐oxides with various reagents under appropriate activation conditions. This review seeks to comprehensively document the available synthetic methods for introducing functional groups at the C2 position of pyridines and quinolines. In this work, we highlight recent developments in the C2‐functionalization of pyridine and quinoline N‐oxides and address both the mechanisms and regioselectivity of the reactions. We also describe the pathways and reactive species involved in these processes and highlight a number of medically relevant nitrogen heteroaromatics.
中文翻译:

使用N氧化物化学合成C2功能化吡啶和喹啉的最新进展
尽管最近在直接CH-H-官能化的杂志方面取得了显着进展,但由于缺乏可及的官能团(主要是基于碳的)和区域选择性差,其应用仍然受到限制。相反,在适当的活化条件下,用各种试剂处理易得的N-氧化物,可以轻松合成C2-官能化的吡啶和喹啉。这篇综述试图全面记录可用于在吡啶和喹啉的C2位引入官能团的合成方法。在这项工作中,我们重点介绍了吡啶和喹啉N在C2功能化方面的最新进展氧化并解决反应的机理和区域选择性。我们还描述了这些过程中涉及的途径和反应性物种,并重点介绍了许多医学上相关的氮杂芳族化合物。
更新日期:2020-10-11
中文翻译:

使用N氧化物化学合成C2功能化吡啶和喹啉的最新进展
尽管最近在直接CH-H-官能化的杂志方面取得了显着进展,但由于缺乏可及的官能团(主要是基于碳的)和区域选择性差,其应用仍然受到限制。相反,在适当的活化条件下,用各种试剂处理易得的N-氧化物,可以轻松合成C2-官能化的吡啶和喹啉。这篇综述试图全面记录可用于在吡啶和喹啉的C2位引入官能团的合成方法。在这项工作中,我们重点介绍了吡啶和喹啉N在C2功能化方面的最新进展氧化并解决反应的机理和区域选择性。我们还描述了这些过程中涉及的途径和反应性物种,并重点介绍了许多医学上相关的氮杂芳族化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号