当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem Annulations of Propargylic Alcohols to Indole Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-11 , DOI: 10.1002/adsc.202000930 Xiao‐Yan Liu 1 , Yun‐Lin Liu 2 , Long Chen 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-11 , DOI: 10.1002/adsc.202000930 Xiao‐Yan Liu 1 , Yun‐Lin Liu 2 , Long Chen 1
Affiliation
|
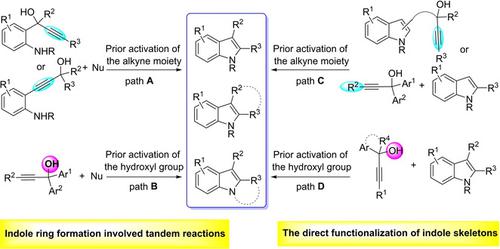
Indole derivatives are important heterocycles in organic synthesis for serving as privileged building blocks for functional material and as key components in a lot of bioactive compounds. Propargylic alcohols, bearing alkynyl and hydroxyl functional groups, have emerged as promising feedstock materials for the construction of carbo‐ and heterocycles. Especially, in the last decade, the Lewis or Brønsted acid catalysed tandem annulations of propargylic alcohols to build structurally diverse indole derivatives have been well‐investigated. In this review, we summarize two main synthetic strategies toward indole derivatives via the cascade reactions of propargylic alcohols: indole‐ring formation involved tandem reactions and the direct function‐alization of indole skeletons. We hope this review would help to develop new and more efficient protocols for the synthesis of indole‐included N‐heterocycles.
中文翻译:

炔丙基醇与吲哚衍生物的串联
吲哚衍生物是有机合成中的重要杂环,可作为功能材料的特权构件和许多生物活性化合物的关键组分。带有炔基和羟基官能团的炔丙醇已成为有前途的原料,可用于构建碳环和杂环。特别是在过去的十年中,对路易斯酸或布朗斯台德酸催化的炔丙醇串联环化以构建结构多样的吲哚衍生物进行了深入研究。在这次审查中,我们总结了向吲哚衍生物的两个主要合成策略通过炔丙醇的级联反应:吲哚环的形成涉及串联反应和吲哚骨架的直接官能化。我们希望这项审查将有助于开发新的和更有效的协议,以合成包含吲哚的N杂环。
更新日期:2020-12-08
中文翻译:

炔丙基醇与吲哚衍生物的串联
吲哚衍生物是有机合成中的重要杂环,可作为功能材料的特权构件和许多生物活性化合物的关键组分。带有炔基和羟基官能团的炔丙醇已成为有前途的原料,可用于构建碳环和杂环。特别是在过去的十年中,对路易斯酸或布朗斯台德酸催化的炔丙醇串联环化以构建结构多样的吲哚衍生物进行了深入研究。在这次审查中,我们总结了向吲哚衍生物的两个主要合成策略通过炔丙醇的级联反应:吲哚环的形成涉及串联反应和吲哚骨架的直接官能化。我们希望这项审查将有助于开发新的和更有效的协议,以合成包含吲哚的N杂环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号