当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium‐Catalyzed Aminomethylation of Nitrodienes and Dienones via Double C—N Bond Activation
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-10-12 , DOI: 10.1002/cjoc.202000184 Bangkui Yu 1 , Bao Gao 1 , Xuexia Zhang 1 , Haocheng Zhang 1 , Hanmin Huang 1, 2
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-10-12 , DOI: 10.1002/cjoc.202000184 Bangkui Yu 1 , Bao Gao 1 , Xuexia Zhang 1 , Haocheng Zhang 1 , Hanmin Huang 1, 2
Affiliation
|
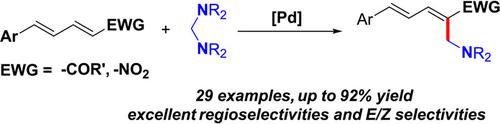
A new strategy for the generation of the active Pd‐alkyl species from aminal via C—N bond activation has been established, in which the formation of zwitterionic intermediate through aza‐Michael addition of aminal to nitrodienes or dienones is identified as a key step for the activation of the C—N bond. The efficient strategy has enabled a new palladium‐catalyzed α‐aminomethylation of nitrodienes and dienones via double C—N bond activation. The scope and versatility of the reaction were demonstrated and a broad range of substrates bearing electron‐donating and ‐withdrawing groups on the aromatic rings were all compatible with this reaction to furnish the desired α‐aminomethylated products in moderate to good yields with excellent regioselectivities and E/Z selectivities.
中文翻译:

钯通过双C-N键活化催化硝基二烯和二烯酮的氨甲基化
从缩醛胺中活性的Pd-烷基物种的产生的新策略通过C-N键活化已经建立,在该两性离子中间体通过氮杂-迈克尔加成的缩醛胺,以nitrodienes或二烯酮的形成被识别为用于一个关键步骤C-N键的活化。有效的策略通过双CN键活化,实现了新的钯催化的硝基二烯和二酮的α-氨基甲基化。证明了该反应的范围和多功能性,并且在芳族环上带有给电子和吸电子基团的多种底物均与该反应相容,从而以中等至良好的收率提供了所需的α-氨基甲基化产物,并具有优异的区域选择性和选择性。E /Z选择性。
更新日期:2020-10-12

中文翻译:

钯通过双C-N键活化催化硝基二烯和二烯酮的氨甲基化
从缩醛胺中活性的Pd-烷基物种的产生的新策略通过C-N键活化已经建立,在该两性离子中间体通过氮杂-迈克尔加成的缩醛胺,以nitrodienes或二烯酮的形成被识别为用于一个关键步骤C-N键的活化。有效的策略通过双CN键活化,实现了新的钯催化的硝基二烯和二酮的α-氨基甲基化。证明了该反应的范围和多功能性,并且在芳族环上带有给电子和吸电子基团的多种底物均与该反应相容,从而以中等至良好的收率提供了所需的α-氨基甲基化产物,并具有优异的区域选择性和选择性。E /Z选择性。












































 京公网安备 11010802027423号
京公网安备 11010802027423号