Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
USP38 Couples Histone Ubiquitination and Methylation via KDM5B to Resolve Inflammation
Advanced Science ( IF 14.3 ) Pub Date : 2020-10-11 , DOI: 10.1002/advs.202002680 Zhiyao Zhao 1, 2 , Zexiong Su 1 , Puping Liang 1 , Di Liu 1 , Shuai Yang 1 , Yaoxing Wu 1 , Ling Ma 1 , Junyan Feng 1 , Xiya Zhang 1 , Chenglei Wu 1 , Junjiu Huang 1 , Jun Cui 1
Advanced Science ( IF 14.3 ) Pub Date : 2020-10-11 , DOI: 10.1002/advs.202002680 Zhiyao Zhao 1, 2 , Zexiong Su 1 , Puping Liang 1 , Di Liu 1 , Shuai Yang 1 , Yaoxing Wu 1 , Ling Ma 1 , Junyan Feng 1 , Xiya Zhang 1 , Chenglei Wu 1 , Junjiu Huang 1 , Jun Cui 1
Affiliation
|
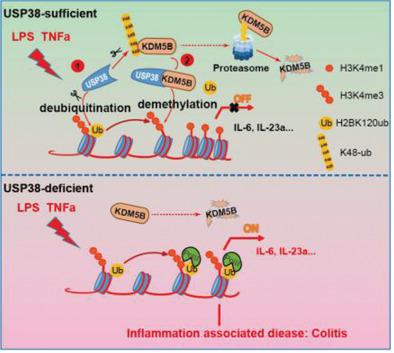
Chromatin modifications, such as histone acetylation, ubiquitination, and methylation, play fundamental roles in maintaining chromatin architecture and regulating gene transcription. Although their crosstalk in chromatin remodeling has been gradually uncovered, the functional relationship between histone ubiquitination and methylation in regulating immunity and inflammation remains unclear. Here, it is reported that USP38 is a novel histone deubiquitinase that works together with the histone H3K4 modifier KDM5B to orchestrate inflammatory responses. USP38 specifically removes the monoubiquitin on H2B at lysine 120, which functions as a prerequisite for the subsequent recruitment of demethylase KDM5B to the promoters of proinflammatory cytokines Il6 and Il23a during LPS stimulation. KDM5B in turn inhibits the binding of NF‐κB transcription factors to the Il6 and Il23a promoters by reducing H3K4 trimethylation. Furthermore, USP38 can bind to KDM5B and prevent it from proteasomal degradation, which further enhances the function of KDM5B in the regulation of inflammation‐related genes. Loss of Usp38 in mice markedly enhances susceptibility to endotoxin shock and acute colitis, and these mice display a more severe inflammatory phenotype compared to wild‐type mice. The studies identify USP38‐KDM5B as a distinct chromatin modification complex that restrains inflammatory responses through manipulating the crosstalk of histone ubiquitination and methylation.
中文翻译:

USP38 通过 KDM5B 耦合组蛋白泛素化和甲基化来解决炎症
染色质修饰,例如组蛋白乙酰化、泛素化和甲基化,在维持染色质结构和调节基因转录方面发挥着重要作用。尽管它们在染色质重塑中的串扰已逐渐被揭示,但组蛋白泛素化和甲基化在调节免疫和炎症中的功能关系仍不清楚。据报道,USP38 是一种新型组蛋白去泛素酶,可与组蛋白 H3K4 修饰剂 KDM5B 一起协调炎症反应。USP38 特异性去除 H2B 赖氨酸 120 处的单泛素,这是随后在 LPS 刺激期间将去甲基酶 KDM5B 募集至促炎细胞因子Il6和Il23a启动子的先决条件。KDM5B 反过来通过减少 H3K4 三甲基化来抑制 NF- κ B 转录因子与Il6和Il23a启动子的结合。此外,USP38可以与KDM5B结合并阻止其蛋白酶体降解,从而进一步增强KDM5B在炎症相关基因调控中的功能。小鼠中Usp38的缺失显着增强了对内毒素休克和急性结肠炎的易感性,并且与野生型小鼠相比,这些小鼠表现出更严重的炎症表型。研究确定 USP38-KDM5B 是一种独特的染色质修饰复合物,可通过操纵组蛋白泛素化和甲基化的串扰来抑制炎症反应。
更新日期:2020-11-19
中文翻译:

USP38 通过 KDM5B 耦合组蛋白泛素化和甲基化来解决炎症
染色质修饰,例如组蛋白乙酰化、泛素化和甲基化,在维持染色质结构和调节基因转录方面发挥着重要作用。尽管它们在染色质重塑中的串扰已逐渐被揭示,但组蛋白泛素化和甲基化在调节免疫和炎症中的功能关系仍不清楚。据报道,USP38 是一种新型组蛋白去泛素酶,可与组蛋白 H3K4 修饰剂 KDM5B 一起协调炎症反应。USP38 特异性去除 H2B 赖氨酸 120 处的单泛素,这是随后在 LPS 刺激期间将去甲基酶 KDM5B 募集至促炎细胞因子Il6和Il23a启动子的先决条件。KDM5B 反过来通过减少 H3K4 三甲基化来抑制 NF- κ B 转录因子与Il6和Il23a启动子的结合。此外,USP38可以与KDM5B结合并阻止其蛋白酶体降解,从而进一步增强KDM5B在炎症相关基因调控中的功能。小鼠中Usp38的缺失显着增强了对内毒素休克和急性结肠炎的易感性,并且与野生型小鼠相比,这些小鼠表现出更严重的炎症表型。研究确定 USP38-KDM5B 是一种独特的染色质修饰复合物,可通过操纵组蛋白泛素化和甲基化的串扰来抑制炎症反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号