当前位置:
X-MOL 学术
›
Mater. Today Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formulation and molecular docking simulation study of luliconazole nanosuspension–based nanogel for transdermal drug delivery using modified polymer
Materials Today Chemistry ( IF 6.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.mtchem.2020.100364 Mohd Sayeed Shaikh , Mayura A. Kale
Materials Today Chemistry ( IF 6.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.mtchem.2020.100364 Mohd Sayeed Shaikh , Mayura A. Kale
|
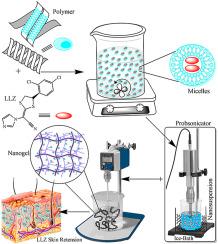
Abstract The purpose of study was to formulate nanosuspension-based nanogel of luliconazole (LLZ) for transdermal delivery to enhance its skin retention and effectiveness using modified starch ester. Nanosuspensions show promising results with size of 369.1–745.4 nm having PDI 0.193–0.344 and zeta potential 22–45 mV. These nanosuspensions form micelles and hydrophobic core of it provides the reservoir for LLZ with better drug loading and binding interaction. Drug loading was confirmed by percent drug entrapment efficiency (PDEE) and PDI. Molecular docking simulation (MDS) provides detail insight of LLZ polymer complexation at hydrophobic cavity of micelles and revealed that there was binding between drug and polymer in aqueous milieu having interaction energy ranges from −7.1 to −6.0 kcal/mol. Nanosuspensions so made were incorporated into gel by using Carbopol 934 ® and tested for % drug content, spreadability, pH, and viscosity with ranges of 101.62–97.71, 28.94–34.38 (gcm/s), 6.91–7.21, and 4802.62–9461.83 (cp), respectively. Nanogel also evaluated for stability and skin permeation study using human cadaver skin (HCS). In vitro skin permeation study indicated that the amount of LLZ permeated through skin from nanogel (71.042–83.818 μgcm −2) was higher than standard cream (70.085 μgcm −2). Nanogel increased the accumulation of LLZ in HCS ~3 times than standard cream. The transdermal flux was greater for standard cream (123.79 μgcm −2), whereas smaller for nanogel (50.394–82.743 μgcm −2) due to skin retention. Nanosuspension-based gel are able to especially favor LLZ accumulation into skin, provide better drug loading, improve stability, and efficacy. Thus, targeting older antibiotics such as LLZ and formulating into nanosystem utilized to expand its usefulness to physicians to treat illnesses caused by resistant fungal strains.
中文翻译:

基于卢立康唑纳米混悬剂的改性聚合物透皮给药纳米凝胶的配方及分子对接模拟研究
摘要 本研究的目的是配制基于纳米混悬剂的卢立康唑纳米凝胶(LLZ)用于透皮给药,以使用改性淀粉酯增强其皮肤保留和有效性。纳米悬浮液显示出有希望的结果,尺寸为 369.1-745.4 nm,PDI 0.193-0.344,zeta 电位 22-45 mV。这些纳米混悬液形成胶束,其疏水核心为 LLZ 提供了更好的载药和结合相互作用的储库。载药量由药物包封率 (PDEE) 和 PDI 百分比确定。分子对接模拟 (MDS) 提供了胶束疏水腔中 LLZ 聚合物络合的详细信息,并揭示了药物和聚合物在相互作用能范围为 -7.1 至 -6.0 kcal/mol 的水性环境中的结合。使用 Carbopol 934 ® 将如此制备的纳米混悬液掺入凝胶中,并测试药物含量百分比、铺展性、pH 值和粘度,范围为 101.62–97.71、28.94–34.38 (gcm/s)、6.91–7.21 和 4802.62–946 cp),分别。Nanogel 还使用人类尸体皮肤 (HCS) 评估了稳定性和皮肤渗透研究。体外皮肤渗透研究表明,从纳米凝胶渗透皮肤的 LLZ 量(71.042–83.818 μgcm -2)高于标准乳膏(70.085 μgcm -2)。Nanogel 增加了 HCS 中 LLZ 的积累,是标准乳膏的 3 倍。由于皮肤滞留,标准乳膏的透皮通量较大(123.79 μgcm -2),而纳米凝胶的透皮通量较小(50.394–82.743 μgcm -2)。基于纳米悬浮液的凝胶能够特别有利于 LLZ 在皮肤中的积累,提供更好的载药量,提高稳定性和疗效。因此,针对诸如 LLZ 之类的旧抗生素并将其配制成纳米系统,以扩大其对医生的用途,以治疗由抗药性真菌菌株引起的疾病。
更新日期:2020-12-01
中文翻译:

基于卢立康唑纳米混悬剂的改性聚合物透皮给药纳米凝胶的配方及分子对接模拟研究
摘要 本研究的目的是配制基于纳米混悬剂的卢立康唑纳米凝胶(LLZ)用于透皮给药,以使用改性淀粉酯增强其皮肤保留和有效性。纳米悬浮液显示出有希望的结果,尺寸为 369.1-745.4 nm,PDI 0.193-0.344,zeta 电位 22-45 mV。这些纳米混悬液形成胶束,其疏水核心为 LLZ 提供了更好的载药和结合相互作用的储库。载药量由药物包封率 (PDEE) 和 PDI 百分比确定。分子对接模拟 (MDS) 提供了胶束疏水腔中 LLZ 聚合物络合的详细信息,并揭示了药物和聚合物在相互作用能范围为 -7.1 至 -6.0 kcal/mol 的水性环境中的结合。使用 Carbopol 934 ® 将如此制备的纳米混悬液掺入凝胶中,并测试药物含量百分比、铺展性、pH 值和粘度,范围为 101.62–97.71、28.94–34.38 (gcm/s)、6.91–7.21 和 4802.62–946 cp),分别。Nanogel 还使用人类尸体皮肤 (HCS) 评估了稳定性和皮肤渗透研究。体外皮肤渗透研究表明,从纳米凝胶渗透皮肤的 LLZ 量(71.042–83.818 μgcm -2)高于标准乳膏(70.085 μgcm -2)。Nanogel 增加了 HCS 中 LLZ 的积累,是标准乳膏的 3 倍。由于皮肤滞留,标准乳膏的透皮通量较大(123.79 μgcm -2),而纳米凝胶的透皮通量较小(50.394–82.743 μgcm -2)。基于纳米悬浮液的凝胶能够特别有利于 LLZ 在皮肤中的积累,提供更好的载药量,提高稳定性和疗效。因此,针对诸如 LLZ 之类的旧抗生素并将其配制成纳米系统,以扩大其对医生的用途,以治疗由抗药性真菌菌株引起的疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号