Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-10-12 , DOI: 10.1016/j.jmb.2020.09.020 Christopher T Nordyke 1 , Yasin M Ahmed 2 , Ryan Z Puterbaugh 1 , Grant R Bowman 2 , Krisztina Varga 1
|
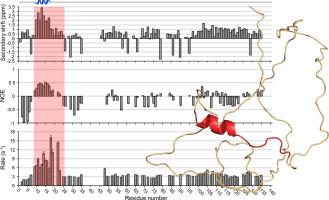
The polar organizing protein Z (PopZ) is necessary for the formation of three-dimensional microdomains at the cell poles in Caulobacter crescentus, where it functions as a hub protein that recruits multiple regulatory proteins from the cytoplasm. Although a large portion of the protein is predicted to be natively unstructured, in reconstituted systems PopZ can self-assemble into a macromolecular scaffold that directly binds to at least ten different proteins. Here we report the solution NMR structure of PopZΔ134–177, a truncated form of PopZ that does not self-assemble but retains the ability to interact with heterologous proteins. We show that the unbound form of PopZΔ134–177 is unstructured in solution, with the exception of a small amphipathic α-helix in residues M10-I17, which is included within a highly conserved region near the N-terminal. In applying NMR techniques to map the interactions between PopZΔ134–177 and one of its binding partners, RcdA, we find evidence that the α-helix and adjoining amino acids extending to position E23 serve as the core of the binding motif. Consistent with this, a point mutation at position I17 severely compromises binding. Our results show that a partially structured Molecular Recognition Feature (MoRF) within an intrinsically disordered domain of PopZ contributes to the assembly of polar microdomains, revealing a structural basis for complex network assembly in Alphaproteobacteria that is analogous to those formed by intrinsically disordered hub proteins in other kingdoms.
中文翻译:

内在无序的细菌极性组织蛋白 Z,PopZ,通过 N 端分子识别特征与蛋白质结合伙伴相互作用
极性组织蛋白 Z (PopZ) 是在新月形茎杆菌的细胞两极形成三维微结构域所必需的,它在其中作为中枢蛋白发挥作用,从细胞质中募集多种调节蛋白。尽管预计大部分蛋白质是天然非结构化的,但在重组系统中,PopZ 可以自组装成直接结合至少十种不同蛋白质的大分子支架。在这里,我们报告了 PopZ Δ134-177的溶液 NMR 结构,这是一种截断形式的 PopZ,不会自组装但保留与异源蛋白质相互作用的能力。我们证明了 PopZ Δ134-177的未结合形式在溶液中是非结构化的,除了残基 M10-I17 中的一个小两亲性 α-螺旋,它包含在 N 末端附近的高度保守区域内。应用 NMR 技术绘制 PopZ Δ134-177之间的相互作用和它的结合伙伴之一,RcdA,我们发现了延伸到 E23 位的 α-螺旋和相邻氨基酸作为结合基序核心的证据。与此一致,位置 I17 的点突变严重损害了结合。我们的研究结果表明,PopZ 本质上无序结构域内的部分结构化分子识别特征 (MoRF) 有助于极性微域的组装,揭示了 Alphaproteobacteria 中复杂网络组装的结构基础,类似于由本质上无序的枢纽蛋白形成的结构基础。其他王国。











































 京公网安备 11010802027423号
京公网安备 11010802027423号