当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A porous multifunctional and magnetic layered graphene oxide/3D mesoporous MOF nanocomposite for rapid adsorption of uranium(VI) from aqueous solutions
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jiec.2020.10.008 Asma Amini , Mostafa Khajeh , Ali Reza Oveisi , Saba Daliran , Mansour Ghaffari-Moghaddam , Hojat Samareh Delarami
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jiec.2020.10.008 Asma Amini , Mostafa Khajeh , Ali Reza Oveisi , Saba Daliran , Mansour Ghaffari-Moghaddam , Hojat Samareh Delarami
|
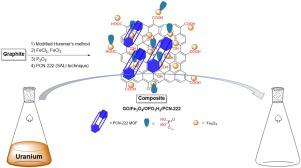
Abstract A novel adsorbent, GO/Fe3O4/OPO3H2/PCN-222, was successfully synthesized via graphene oxide (GO) modification with magnetic particles, phosphorous-containing groups, and a mesoporous Zr-MOF, PCN-222 (PCN stands for Porous Coordination Network), respectively, to give the nominal composite. The last step was through a solvent-assisted ligand incorporation technique. Morphological, structural, and physicochemical properties of the hybrid material was assessed by FT-IR, PXRD, SEM/EDX, BET surface area, VSM, TGA/DSC, UV–vis DRS, and ICP-OES analyses. This solid was then used for dispersive solid phase extraction of uranium ions dissolved in water. Several parameters including pH of solution, extraction and desorption times, amount of adsorbent, and type and concentration of elution solvent were investigated and optimized. The maximum adsorption capacity of adsorbent was found to be 416.7 mg g−1 (pH, 6.2; amount of adsorbent, 5.0 mg; extraction time, 3.0 min) beyond what was achievable with the individual components. In addition, various coordination modes between the multifunctional adsorbent and uranyl ions were investigated by DFT calculations in details, revealing some favorable non-covalent cation–π interaction and strong binding of free-base porphyrin, carboxyl and phosphorous-containing groups to the uranium ions. Under optimized conditions, high determination coefficient (R2 = 0.9994) was obtained and limit of detection and relative standard deviation were found 0.9 μg L−1 and 2.7%, respectively.
中文翻译:

一种多孔多功能磁性层状氧化石墨烯/3D 介孔 MOF 纳米复合材料,用于从水溶液中快速吸附铀(VI)
摘要 通过用磁性颗粒、含磷基团和介孔 Zr-MOF、PCN-222(PCN 代表多孔配位)修饰氧化石墨烯(GO),成功合成了一种新型吸附剂 GO/Fe3O4/OPO3H2/PCN-222。网络),分别给出名义复合。最后一步是通过溶剂辅助配体掺入技术。通过 FT-IR、PXRD、SEM/EDX、BET 表面积、VSM、TGA/DSC、UV-vis DRS 和 ICP-OES 分析评估混合材料的形态、结构和物理化学性质。然后将该固体用于溶解在水中的铀离子的分散固相萃取。研究和优化了几个参数,包括溶液的 pH 值、萃取和解吸时间、吸附剂的量以及洗脱溶剂的类型和浓度。发现吸附剂的最大吸附容量为 416.7 mg g-1(pH 值,6.2;吸附剂的量,5.0 mg;提取时间,3.0 分钟),超出了单个组分的吸附能力。此外,通过DFT计算详细研究了多功能吸附剂与铀酰离子之间的各种配位模式,揭示了一些有利的非共价阳离子-π相互作用以及游离碱卟啉、羧基和含磷基团与铀离子的强结合. 在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。0 分钟)超出了单个组件可实现的目标。此外,通过DFT计算详细研究了多功能吸附剂与铀酰离子之间的各种配位模式,揭示了一些有利的非共价阳离子-π相互作用以及游离碱卟啉、羧基和含磷基团与铀离子的强结合. 在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。0 分钟)超出了单个组件可实现的目标。此外,通过DFT计算详细研究了多功能吸附剂与铀酰离子之间的各种配位模式,揭示了一些有利的非共价阳离子-π相互作用以及游离碱卟啉、羧基和含磷基团与铀离子的强结合. 在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。含羧基和磷的基团加到铀离子上。在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。含羧基和磷的基团加到铀离子上。在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。
更新日期:2021-01-01
中文翻译:

一种多孔多功能磁性层状氧化石墨烯/3D 介孔 MOF 纳米复合材料,用于从水溶液中快速吸附铀(VI)
摘要 通过用磁性颗粒、含磷基团和介孔 Zr-MOF、PCN-222(PCN 代表多孔配位)修饰氧化石墨烯(GO),成功合成了一种新型吸附剂 GO/Fe3O4/OPO3H2/PCN-222。网络),分别给出名义复合。最后一步是通过溶剂辅助配体掺入技术。通过 FT-IR、PXRD、SEM/EDX、BET 表面积、VSM、TGA/DSC、UV-vis DRS 和 ICP-OES 分析评估混合材料的形态、结构和物理化学性质。然后将该固体用于溶解在水中的铀离子的分散固相萃取。研究和优化了几个参数,包括溶液的 pH 值、萃取和解吸时间、吸附剂的量以及洗脱溶剂的类型和浓度。发现吸附剂的最大吸附容量为 416.7 mg g-1(pH 值,6.2;吸附剂的量,5.0 mg;提取时间,3.0 分钟),超出了单个组分的吸附能力。此外,通过DFT计算详细研究了多功能吸附剂与铀酰离子之间的各种配位模式,揭示了一些有利的非共价阳离子-π相互作用以及游离碱卟啉、羧基和含磷基团与铀离子的强结合. 在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。0 分钟)超出了单个组件可实现的目标。此外,通过DFT计算详细研究了多功能吸附剂与铀酰离子之间的各种配位模式,揭示了一些有利的非共价阳离子-π相互作用以及游离碱卟啉、羧基和含磷基团与铀离子的强结合. 在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。0 分钟)超出了单个组件可实现的目标。此外,通过DFT计算详细研究了多功能吸附剂与铀酰离子之间的各种配位模式,揭示了一些有利的非共价阳离子-π相互作用以及游离碱卟啉、羧基和含磷基团与铀离子的强结合. 在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。含羧基和磷的基团加到铀离子上。在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。含羧基和磷的基团加到铀离子上。在优化条件下,获得了高测定系数 (R2 = 0.9994),检测限和相对标准偏差分别为 0.9 μg L-1 和 2.7%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号