Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-10-12 , DOI: 10.1016/j.bioorg.2020.104365 Fouzia Naz , Kanwal , Mehreen Latif , Uzma Salar , Khalid Mohammed Khan , Mariya al-Rashida , Irfan Ali , Basharat Ali , Muhammad Taha , Shahnaz Perveen
|
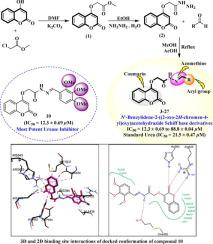
Urease enzyme is responsible to catalyze the hydrolysis of urea into carbamate and ammonia. Then carbamate hydrolyzed to ammonia and carbon dioxide. Excess release of ammonia leads to increase pH in stomach that actually encourages the survival of Helicobacter pylori. H. pylori involves in various disorders most commonly peptic ulcer, pyelonephritis, hepatic coma, kidney stone formation, urolithiasis, and encephalopathy. Apart from many pharmacological properties, coumarin and Schiff bases are known to possess urease inhibitory activity. Therefore, these two pharmacologically important scaffolds are combined into single hybrid molecules to assess their potential as urease inhibitors. For this aim, N′-benzylidene-2-((2-oxo-2H-chromen-4-yl)oxy)acetohydrazide Schiff base derivatives 3–27 were synthesized by following a three step reaction strategy. Structures of all synthetic molecules were characterized by EI-MS, 1H-, and 13C NMR spectroscopic techniques. All molecules were assessed for urease inhibitory activity and found to possess a varying degree of inhibitory potential in the range of IC50 = 12.3 ± 0.69 to 88.8 ± 0.04 μM. Amongst the active analogs, compounds 7 (IC50 = 16.2 ± 0.11 μM), 9 (IC50 = 15.2 ± 0.14 μM), 10 (IC50 = 12.3 ± 0.69 μM), 12 (IC50 = 16.3 ± 0.45 μM), and 15 (IC50 = 17.6 ± 0.28 μM) were identified as potent inhibitors compared to standard urea (IC50 = 21.5 ± 0.47 μM). It is conferred from structure-activity relationship (SAR) that variation in inhibitory activity is due to different substitutions pattern on aryl ring. Moreover, molecular docking studies were carried out to understand the interactions of ligand with the active pocket of urease enzyme.
中文翻译:

4-羟基香豆素基连接的乙酰肼席夫碱作为有效的脲酶抑制剂
尿素酶负责催化尿素水解为氨基甲酸酯和氨。然后氨基甲酸酯水解为氨和二氧化碳。氨的过量释放导致胃pH升高,实际上促进了幽门螺杆菌的生存。幽门螺杆菌涉及多种疾病,最常见的是消化性溃疡,肾盂肾炎,肝昏迷,肾结石形成,尿石症和脑病。除了许多药理特性,香豆素和席夫碱还具有脲酶抑制活性。因此,将这两个在药理上重要的支架组合成单个杂合分子,以评估其作为脲酶抑制剂的潜力。为此,N'-亚苄基-2-((2-oxo-2 H通过三步反应策略合成了-chromen-4-yl)oxy)乙酰肼Schiff碱衍生物3 – 27。所有合成分子的结构均通过EI-MS,1 H-和13 C NMR光谱技术表征。评估所有分子的脲酶抑制活性,发现它们具有不同程度的抑制潜能,范围为IC 50 = 12.3±0.69至88.8±0.04μM。在活性类似物中,化合物7(IC 50 = 16.2±0.11μM),9(IC 50 = 15.2±0.14μM),10(IC 50 = 12.3±0.69μM),12(IC 与标准尿素(IC 50 = 21.5±0.47μM)相比,50 = 16.3±0.45μM)和15(IC 50 = 17.6±0.28μM)被确定为有效抑制剂。从结构活性关系(SAR)得出抑制活性的变化是由于芳基环上的不同取代方式所致。此外,进行了分子对接研究以了解配体与脲酶活性口袋的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号