当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the lipid production mechanism in Euglena gracilis with a fast-response AIEgen bioprobe, DPAS
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2020-10-09 , DOI: 10.1039/d0qm00621a AHM Mohsinul Reza 1, 1, 2, 3, 4 , Yabin Zhou 1, 2, 3 , Javad Tavakoli 1, 2, 3, 4, 5 , Youhong Tang 1, 1, 2, 3, 4 , Jianguang Qin 1, 2, 3
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2020-10-09 , DOI: 10.1039/d0qm00621a AHM Mohsinul Reza 1, 1, 2, 3, 4 , Yabin Zhou 1, 2, 3 , Javad Tavakoli 1, 2, 3, 4, 5 , Youhong Tang 1, 1, 2, 3, 4 , Jianguang Qin 1, 2, 3
Affiliation
|
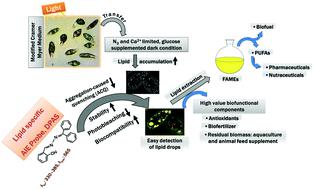
Lipid bodies are lipid-rich organelles that can regulate the storage of neutral lipids as energy sources in organisms. Visualisation of lipid droplets is an effective approach to understand lipid dynamics in microalgae. This study explores the required environmental conditions to yield lipid in a microalgal species Euglena gracilis as the biofunctional component using a lipid-specific aggregation-induced emission fluorogen, DPAS (C20H16N2O), and compares it to the commercial lipid staining probe BODIPY for visualising the lipid production in vivo. Five treatments are investigated for lipid production: (1) modified Cramer–Myers medium (MCM), (2) MCM without nitrogen (−), (3) MCM without nitrogen (−) and calcium (−), (4) MCM without nitrogen (−) and calcium (−), but with glucose (+), and (5) MCM without nitrogen (−) and calcium (−), but with glucose (+). Illumination was continuous at a rate of 70 mmol photons per m−2 s−1 in all treatments except with no light for treatment of 5. Distinct lipid droplets are labelled with DPAS and detected by confocal microscopy and flow cytometry to clarify the understanding of the lipid enrichment mechanism under various conditions. Treatment 1 indicates low lipid production in E. gracilis under an autotrophic condition. DPAS benefits from a very low background signal, and therefore, it is more sensitive than BODIPY for semiquantitative in vivo fluorescence measurements. Co-staining in the presence of BODIPY and chlorophyll also indicates that DPAS is suitable for multicolour imaging with red and green fluorophores. The present study demonstrates that DPAS is a highly effective biocompatible and photostable fluorophore for rapid and sensitive visualisation of lipid droplets. This novel staining method could be used to screen microalgae that have a potential to produce lipid droplets as a health supplement for humans.
中文翻译:

通过快速响应的AIEgen生物探针DPAS了解细叶藻的脂质产生机理
脂质体是富含脂质的细胞器,可以调节中性脂质作为生物体内能源的储存。脂滴的可视化是了解微藻中脂质动态的有效方法。这项研究探索了使用脂类特异性聚集诱导的发射氟离子DPAS(C 20 H 16 N 2 O)来产生微藻物种Euglena gracilis中的脂质作为生物功能成分的必要环境条件,并将其与商业脂质染色进行了比较。探针BODIPY用于可视化体内脂质的产生。研究了五种处理脂质产生的方法:(1)改良的Cramer-Myers培养基(MCM),(2)不带氮(-)的MCM,(3)不带氮(-)和钙(-)的MCM,(4)不带氮的MCM氮(-)和钙(-),但有葡萄糖(+),以及(5)MCM没有氮(-)和钙(-),但有葡萄糖(+)。在所有处理中,以70 mmol光子/ m -2 s -1的速率连续照射,除了不使用5种光照射外,均用DPAS标记不同的脂质滴,并通过共聚焦显微镜和流式细胞仪进行检测,以阐明对在各种条件下脂质的富集机理。处理1表示细纹肠球菌的脂质生成量低在自养条件下。DPAS得益于极低的背景信号,因此,对于半定量体内荧光测量,它比BODIPY更灵敏。在存在BODIPY和叶绿素的情况下进行共染色也表明DPAS适用于使用红色和绿色荧光团进行多色成像。本研究表明,DPAS是一种高效的生物相容性和光稳定性荧光团,可用于脂质滴的快速灵敏可视化。这种新颖的染色方法可用于筛选微藻,这些微藻具有产生脂质小滴的潜力,可作为人类的健康补品。
更新日期:2020-11-03
中文翻译:

通过快速响应的AIEgen生物探针DPAS了解细叶藻的脂质产生机理
脂质体是富含脂质的细胞器,可以调节中性脂质作为生物体内能源的储存。脂滴的可视化是了解微藻中脂质动态的有效方法。这项研究探索了使用脂类特异性聚集诱导的发射氟离子DPAS(C 20 H 16 N 2 O)来产生微藻物种Euglena gracilis中的脂质作为生物功能成分的必要环境条件,并将其与商业脂质染色进行了比较。探针BODIPY用于可视化体内脂质的产生。研究了五种处理脂质产生的方法:(1)改良的Cramer-Myers培养基(MCM),(2)不带氮(-)的MCM,(3)不带氮(-)和钙(-)的MCM,(4)不带氮的MCM氮(-)和钙(-),但有葡萄糖(+),以及(5)MCM没有氮(-)和钙(-),但有葡萄糖(+)。在所有处理中,以70 mmol光子/ m -2 s -1的速率连续照射,除了不使用5种光照射外,均用DPAS标记不同的脂质滴,并通过共聚焦显微镜和流式细胞仪进行检测,以阐明对在各种条件下脂质的富集机理。处理1表示细纹肠球菌的脂质生成量低在自养条件下。DPAS得益于极低的背景信号,因此,对于半定量体内荧光测量,它比BODIPY更灵敏。在存在BODIPY和叶绿素的情况下进行共染色也表明DPAS适用于使用红色和绿色荧光团进行多色成像。本研究表明,DPAS是一种高效的生物相容性和光稳定性荧光团,可用于脂质滴的快速灵敏可视化。这种新颖的染色方法可用于筛选微藻,这些微藻具有产生脂质小滴的潜力,可作为人类的健康补品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号