当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organic matter influences transformation products of ferrihydrite exposed to sulfide
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-10-09 , DOI: 10.1039/d0en00398k Laurel K. ThomasArrigo 1, 2, 3, 4, 5 , Sylvain Bouchet 1, 2, 3, 4, 5 , Ralf Kaegi 6, 7, 8 , Ruben Kretzschmar 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-10-09 , DOI: 10.1039/d0en00398k Laurel K. ThomasArrigo 1, 2, 3, 4, 5 , Sylvain Bouchet 1, 2, 3, 4, 5 , Ralf Kaegi 6, 7, 8 , Ruben Kretzschmar 1, 2, 3, 4, 5
Affiliation
|
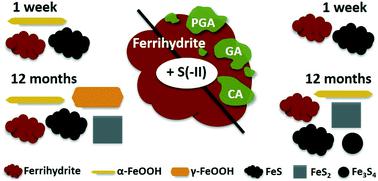
In redox-dynamic environments, sorption to poorly-crystalline, nanometer-sized Fe(III)-(oxyhydr)oxides like ferrihydrite influences the biogeochemical cycling of nutrients and trace elements. Under sulfate-reducing conditions, the reductive dissolution of ferrihydrite leads to the release of associated constituents, which may be re-immobilized via sorption to secondary Fe minerals. To date, studies following the kinetics and transformation pathways of Fe(III)-(oxyhydr)oxides upon exposure to dissolved sulfide (S(−II)) have largely focused on pure Fe minerals. However, in nature, Fe(III)-(oxyhydr)oxides are often found in association with organic matter (OM). Because ferrihydrite–OM associations exhibit characteristics and biogeochemical reactivity differing from those of pure ferrihydrite, in this study, we compared sulfidization kinetics and transformation pathways of a pure ferrihydrite to those of ferrihydrite coprecipitated with contrasting organic ligands; polygalacturonic acid, galacturonic acid, and citric acid (C/Fe molar ratio ∼0.55). Incorporating aqueous- and solid-phase S and Fe speciation analyses (via wet chemistry techniques and S and Fe X-ray absorption spectroscopy) in addition to X-ray diffraction and electron microscopy, we studied both rapid (<7 days) and long-term (12 months) mineral transformations as well as the impact of varying S(−II)/Fe molar ratios at neutral pH. Our results showed that at low S(−II)/Fe molar ratios (=0.1), poorly-crystalline Fe sulfide minerals (e.g. mackinawite) did not form in any (co)precipitate. In contrast, at higher S(−II)/Fe molar ratios (=0.5), mackinawite rapidly precipitated, with higher contributions detected in the coprecipitates than in the pure ferrihydrite. Aging of the samples led to further mineral transformations, including divergent pyrite and greigite precipitation, and an overall increase in the crystallinity of secondary mineral phases. Still, the fraction of residual ferrihydrite at 12 months was higher in the OM-containing coprecipitates, with the most ferrihydrite preservation observed in coprecipitates comprising carboxyl-poor ligands (galacturonic acid and citric acid). This suggests that the presence of OM inhibited S(−II)-induced ferrihydrite mineral transformations and that the composition of the associated OM influenced mineral transformation pathways. Collectively, these results suggest that further studies regarding sulfidization pathways should include OM in order to better represent environmental conditions.
中文翻译:

有机物影响暴露于硫化物的水铁矿的转化产物
在氧化还原动力学环境中,对结晶度低的纳米级Fe(III)-(羟基)氧化物如亚铁酸盐的吸附会影响营养物和微量元素的生物地球化学循环。在硫酸盐还原条件下,水铁矿的还原溶解导致相关成分的释放,这些成分可以通过吸附在次生Fe矿物上而重新固定。迄今为止,关于Fe(III)-(羟基)氧化物暴露于溶解的硫化物(S(-II))的动力学和转化途径的研究主要集中在纯铁矿物质上。然而,在自然界中,Fe(III)-(羟基)氧化物通常与有机物(OM)结合使用。由于水铁矿-OM的结合表现出与纯水铁矿不同的特性和生物地球化学反应性,因此在本研究中,我们比较了纯水铁矿和与相对有机配体共沉淀的水铁矿的硫化动力学和转化途径。聚半乳糖醛酸,半乳糖醛酸和柠檬酸(C / Fe摩尔比约为0.55)。除了X射线衍射和电子显微镜外,还结合了水相和固相S和Fe形态分析(通过湿化学技术以及S和Fe X射线吸收光谱法),我们研究了快速(<7天)和长时学期(12个月)矿物转化以及变化的S(-II)的影响/ Fe在中性pH下的摩尔比。我们的结果表明,在低S(-II)/ Fe摩尔比(= 0.1)下,任何(共)沉淀物中都不会形成低结晶度的Fe硫化物矿物质(例如,麦基钠铁矿)。相反,在较高的S(-II)/ Fe摩尔比(= 0.5)时,马基金矿迅速沉淀,在共沉淀物中检测到的贡献要比纯铁矿中更高。样品的老化导致进一步的矿物转变,包括发散的黄铁矿和钙镁矿沉淀,以及次生矿物相的结晶度整体增加。尽管如此,在含OM的共沉淀物中,在12个月时残留的亚铁水合物的比例更高,在包含贫羧基配体(半乳糖醛酸和柠檬酸)的共沉淀物中,亚铁酸盐的保存最多。这表明OM的存在抑制了S(-II)引起的水铁矿矿物转化以及相关OM的组成影响了矿物转化途径。总的来说,这些结果表明,关于硫化途径的进一步研究应包括OM,以更好地代表环境条件。
更新日期:2020-11-03
中文翻译:

有机物影响暴露于硫化物的水铁矿的转化产物
在氧化还原动力学环境中,对结晶度低的纳米级Fe(III)-(羟基)氧化物如亚铁酸盐的吸附会影响营养物和微量元素的生物地球化学循环。在硫酸盐还原条件下,水铁矿的还原溶解导致相关成分的释放,这些成分可以通过吸附在次生Fe矿物上而重新固定。迄今为止,关于Fe(III)-(羟基)氧化物暴露于溶解的硫化物(S(-II))的动力学和转化途径的研究主要集中在纯铁矿物质上。然而,在自然界中,Fe(III)-(羟基)氧化物通常与有机物(OM)结合使用。由于水铁矿-OM的结合表现出与纯水铁矿不同的特性和生物地球化学反应性,因此在本研究中,我们比较了纯水铁矿和与相对有机配体共沉淀的水铁矿的硫化动力学和转化途径。聚半乳糖醛酸,半乳糖醛酸和柠檬酸(C / Fe摩尔比约为0.55)。除了X射线衍射和电子显微镜外,还结合了水相和固相S和Fe形态分析(通过湿化学技术以及S和Fe X射线吸收光谱法),我们研究了快速(<7天)和长时学期(12个月)矿物转化以及变化的S(-II)的影响/ Fe在中性pH下的摩尔比。我们的结果表明,在低S(-II)/ Fe摩尔比(= 0.1)下,任何(共)沉淀物中都不会形成低结晶度的Fe硫化物矿物质(例如,麦基钠铁矿)。相反,在较高的S(-II)/ Fe摩尔比(= 0.5)时,马基金矿迅速沉淀,在共沉淀物中检测到的贡献要比纯铁矿中更高。样品的老化导致进一步的矿物转变,包括发散的黄铁矿和钙镁矿沉淀,以及次生矿物相的结晶度整体增加。尽管如此,在含OM的共沉淀物中,在12个月时残留的亚铁水合物的比例更高,在包含贫羧基配体(半乳糖醛酸和柠檬酸)的共沉淀物中,亚铁酸盐的保存最多。这表明OM的存在抑制了S(-II)引起的水铁矿矿物转化以及相关OM的组成影响了矿物转化途径。总的来说,这些结果表明,关于硫化途径的进一步研究应包括OM,以更好地代表环境条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号