当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,2-Addition and cycloaddition reactions of niobium bis(imido) and oxo imido complexes
Chemical Science ( IF 7.6 ) Pub Date : 2020-10-09 , DOI: 10.1039/d0sc03489d Jade I Fostvedt 1 , Lauren N Grant 1 , Benjamin M Kriegel 1 , Andreas H Obenhuber 1 , Trevor D Lohrey 1, 2 , Robert G Bergman 1 , John Arnold 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2020-10-09 , DOI: 10.1039/d0sc03489d Jade I Fostvedt 1 , Lauren N Grant 1 , Benjamin M Kriegel 1 , Andreas H Obenhuber 1 , Trevor D Lohrey 1, 2 , Robert G Bergman 1 , John Arnold 1, 2
Affiliation
|
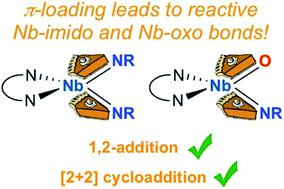
The bis(imido) complexes (BDI)Nb(NtBu)2 and (BDI)Nb(NtBu)(NAr) (BDI = N,N′-bis(2,6-diisopropylphenyl)-3,5-dimethyl-β-diketiminate; Ar = 2,6-diisopropylphenyl) were shown to engage in 1,2-addition and [2 + 2] cycloaddition reactions with a wide variety of substrates. Reaction of the bis(imido) complexes with dihydrogen, silanes, and boranes yielded hydrido-amido-imido complexes via 1,2-addition across Nb-imido π-bonds; some of these complexes were shown to further react via insertion of carbon dioxide to give formate-amido-imido products. Similarly, reaction of (BDI)Nb(NtBu)2 with tert-butylacetylene yielded an acetylide-amido-imido complex. In contrast to these results, many related mono(imido) Nb BDI complexes do not exhibit 1,2-addition reactivity, suggesting that π-loading plays an important role in activating the Nb–N π-bonds toward addition. The same bis(imido) complexes were also shown to engage in [2 + 2] cycloaddition reactions with oxygen- and sulfur-containing heteroallenes to give carbamate- and thiocarbamate-imido complexes: some of these complexes readily dimerized to give bis-μ-sulfido, bis-μ-iminodicarboxylate, and bis-μ-carbonate complexes. The mononuclear carbamate imido complex (BDI)Nb(NAr)(N(tBu)CO2) (12) could be induced to eject tert-butylisocyanate to generate a four-coordinate terminal oxo imido intermediate, which could be trapped as the five-coordinate pyridine or DMAP adduct. The DMAP adducted oxo imido complex (BDI)NbO(NAr)(DMAP) (16) was shown to engage in 1,2-addition of silanes across the Nb-oxo π-bond; this represents a new reaction pathway in group 5 chemistry.
中文翻译:

铌双(亚氨基)和氧代亚氨基配合物的1,2-加成和环加成反应
双(亚氨基)络合物 (BDI)Nb(N t Bu) 2和 (BDI)Nb(N t Bu)(NAr) (BDI = N , N ′-双(2,6-二异丙基苯基)-3,5-二甲基-β-二酮亚胺酯;Ar = 2,6-二异丙基苯基)可与多种底物发生 1,2-加成和 [2 + 2] 环加成反应。双(亚氨基)配合物与二氢、硅烷和硼烷反应,通过Nb-亚氨基 π-键上的 1,2-加成生成氢化-酰胺-亚氨基配合物;其中一些复合物通过插入二氧化碳进一步反应,生成甲酸酯-酰胺基-亚氨基产物。类似地,(BDI)Nb(N t Bu) 2与叔丁基乙炔的反应产生乙炔-酰氨基-亚氨基络合物。与这些结果相反,许多相关的单(亚氨基)Nb BDI 配合物不表现出 1,2-加成反应性,这表明 π-负载在激活 Nb-N π-键加成方面发挥着重要作用。相同的双(亚氨基)络合物也被证明可以与含氧和硫的杂联烯进行[2 + 2]环加成反应,得到氨基甲酸酯-和硫代氨基甲酸酯-亚氨基络合物:其中一些络合物很容易二聚化,得到双-μ-硫基、双-μ-亚氨基二羧酸盐和双-μ-碳酸盐复合物。 单核氨基甲酸酯亚氨基配合物(BDI)Nb(NAr)(N( t Bu)CO 2 ) ( 12 )可以被诱导弹出叔丁基异氰酸酯,生成四配位末端氧代亚氨基中间体,该中间体可以被捕获为五个-配位吡啶或DMAP加合物。 DMAP 加合的氧代酰亚胺络合物 (BDI)NbO(NAr)(DMAP) ( 16 ) 被证明可以在 Nb-氧代 π 键上参与硅烷的 1,2-加成;这代表了第 5 族化学中的新反应途径。
更新日期:2020-10-11
中文翻译:

铌双(亚氨基)和氧代亚氨基配合物的1,2-加成和环加成反应
双(亚氨基)络合物 (BDI)Nb(N t Bu) 2和 (BDI)Nb(N t Bu)(NAr) (BDI = N , N ′-双(2,6-二异丙基苯基)-3,5-二甲基-β-二酮亚胺酯;Ar = 2,6-二异丙基苯基)可与多种底物发生 1,2-加成和 [2 + 2] 环加成反应。双(亚氨基)配合物与二氢、硅烷和硼烷反应,通过Nb-亚氨基 π-键上的 1,2-加成生成氢化-酰胺-亚氨基配合物;其中一些复合物通过插入二氧化碳进一步反应,生成甲酸酯-酰胺基-亚氨基产物。类似地,(BDI)Nb(N t Bu) 2与叔丁基乙炔的反应产生乙炔-酰氨基-亚氨基络合物。与这些结果相反,许多相关的单(亚氨基)Nb BDI 配合物不表现出 1,2-加成反应性,这表明 π-负载在激活 Nb-N π-键加成方面发挥着重要作用。相同的双(亚氨基)络合物也被证明可以与含氧和硫的杂联烯进行[2 + 2]环加成反应,得到氨基甲酸酯-和硫代氨基甲酸酯-亚氨基络合物:其中一些络合物很容易二聚化,得到双-μ-硫基、双-μ-亚氨基二羧酸盐和双-μ-碳酸盐复合物。 单核氨基甲酸酯亚氨基配合物(BDI)Nb(NAr)(N( t Bu)CO 2 ) ( 12 )可以被诱导弹出叔丁基异氰酸酯,生成四配位末端氧代亚氨基中间体,该中间体可以被捕获为五个-配位吡啶或DMAP加合物。 DMAP 加合的氧代酰亚胺络合物 (BDI)NbO(NAr)(DMAP) ( 16 ) 被证明可以在 Nb-氧代 π 键上参与硅烷的 1,2-加成;这代表了第 5 族化学中的新反应途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号