当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Aryl Ligand Identity on Catalytic Performance of Trineopentylphosphine Arylpalladium Complexes in N-Arylation Reactions
Organometallics ( IF 2.8 ) Pub Date : 2020-10-09 , DOI: 10.1021/acs.organomet.0c00140 Huaiyuan Hu 1 , Corrie E. Burlas 1 , Sabrina J. Curley 1 , Tomasz Gruchala 1 , Fengrui Qu 1 , Kevin H. Shaughnessy 1
Organometallics ( IF 2.8 ) Pub Date : 2020-10-09 , DOI: 10.1021/acs.organomet.0c00140 Huaiyuan Hu 1 , Corrie E. Burlas 1 , Sabrina J. Curley 1 , Tomasz Gruchala 1 , Fengrui Qu 1 , Kevin H. Shaughnessy 1
Affiliation
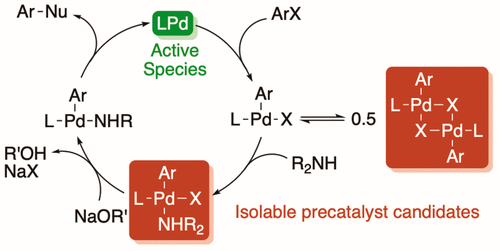
|
A series of air-stable [(Np3P)Pd(Ar)Br]2 (Np = neopentyl) and (Np3P)Pd(Ar)(amine)Br complexes (amine = morpholine and isobutylamine) have been prepared and tested as precatalysts for the coupling of sterically demanding aryl bromides and aniline derivatives. The complexes are more active than the catalyst generated in situ from Pd2(dba)3 and PNp3. Increasing steric demand of the aryl group on palladium correlates with increased catalyst activity. Reactions catalyzed by [(Np3P)Pd(2,6-Me2C6H3)Br]2 occur efficiently at lower temperatures and with similar or higher yields than those using Pd2(dba)3/PNp3. The amine adducts give lower reaction rates and conversion to product than the [(Np3P)Pd(Ar)Br]2 complexes. The lower activity of the amine adducts appears to result from slow base-promoted reductive elimination to generate the catalytically active LPd(0) species.
中文翻译:

芳基配体对N-芳基化反应中三甲苯基膦膦芳基钯配合物催化性能的影响
制备了一系列空气稳定的[(Np 3 P)Pd(Ar)Br] 2(Np =新戊基)和(Np 3 P)Pd(Ar)(胺)Br配合物(胺=吗啉和异丁胺),并且经测试可作为位阻芳基溴化物和苯胺衍生物偶联的前催化剂。该络合物比由Pd 2(dba)3和PNp 3原位产生的催化剂更具活性。钯上芳基空间需求的增加与催化剂活性的提高有关。[(Np 3 P)Pd(2,6-Me 2 C 6 H 3)Br] 2催化的反应与使用Pd 2(dba)3 / PNp 3的那些相比,在更低的温度下以更高的收率有效地发生。与[(Np 3 P)Pd(Ar)Br] 2络合物相比,胺加合物的反应速率和转化率更低。胺加合物的较低活性似乎是由缓慢的碱促进的还原消除产生的,从而产生催化活性的LPd(0)物质。
更新日期:2020-10-27
中文翻译:

芳基配体对N-芳基化反应中三甲苯基膦膦芳基钯配合物催化性能的影响
制备了一系列空气稳定的[(Np 3 P)Pd(Ar)Br] 2(Np =新戊基)和(Np 3 P)Pd(Ar)(胺)Br配合物(胺=吗啉和异丁胺),并且经测试可作为位阻芳基溴化物和苯胺衍生物偶联的前催化剂。该络合物比由Pd 2(dba)3和PNp 3原位产生的催化剂更具活性。钯上芳基空间需求的增加与催化剂活性的提高有关。[(Np 3 P)Pd(2,6-Me 2 C 6 H 3)Br] 2催化的反应与使用Pd 2(dba)3 / PNp 3的那些相比,在更低的温度下以更高的收率有效地发生。与[(Np 3 P)Pd(Ar)Br] 2络合物相比,胺加合物的反应速率和转化率更低。胺加合物的较低活性似乎是由缓慢的碱促进的还原消除产生的,从而产生催化活性的LPd(0)物质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号