当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Enantioselective Radical 1,4-Difunctionalization of 1,3-Enynes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-09 , DOI: 10.1021/jacs.0c06177 Yuehua Zeng 1 , Mong-Feng Chiou 1 , Xiaotao Zhu 1, 2 , Jie Cao 1 , Daqi Lv 1 , Wujun Jian 1 , Yajun Li 1 , Xinhao Zhang 3, 4 , Hongli Bao 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-09 , DOI: 10.1021/jacs.0c06177 Yuehua Zeng 1 , Mong-Feng Chiou 1 , Xiaotao Zhu 1, 2 , Jie Cao 1 , Daqi Lv 1 , Wujun Jian 1 , Yajun Li 1 , Xinhao Zhang 3, 4 , Hongli Bao 1, 2
Affiliation
|
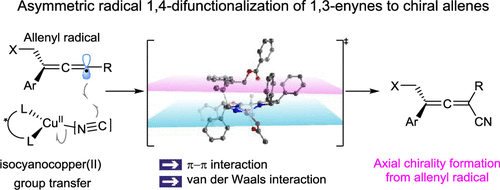
Chiral allenes are important structural motifs frequently found in natural products, pharmaceuticals, and other organic compounds. Asymmetric 1,4-difunctionalization of 1,3-enynes is a promising strategy to construct axial chirality and produce substituted chiral allenes from achiral substrates. However, the previous state of the art in 1,4-difunctionalization of 1,3-enynes focused on the allenyl anion pathway. Because of this, only electrophiles can be introduced into the allene backbones in the second functionalization step, consequently limiting the reaction and allene product types. The development of asymmetric 1,4-difunctionalization of 1,3-enynes via a radical pathway would complement previous methods and support expansion of the toolbox for the synthesis of asymmetric allenes. Herein, we report the first radical enantioselective allene formation via a group transfer pathway in the context of copper-catalyzed radical 1,4-difunctionalization of 1,3-enynes. This method addresses a longstanding unsolved problem in asymmetric radical chemistry, provides an important strategy for stereocontrol with free allenyl radicals, and offers a novel approach to the valuable, but previously inaccessible, chiral allenes. This work should shed light on asymmetric radical reactions and may lead to other enantioselective group transfer reactions.
中文翻译:

铜催化的 1,3-烯炔的对映选择性自由基 1,4-双官能化
手性丙二烯是重要的结构基序,常见于天然产物、药物和其他有机化合物中。1,3-烯炔的不对称1,4-双官能化是构建轴向手性和从非手性底物产生取代的手性丙二烯的一种很有前景的策略。然而,1,3-烯炔的 1,4-双官能化的先前技术状态集中在丙二烯基阴离子途径上。因此,在第二个功能化步骤中只能将亲电子试剂引入丙二烯主链,从而限制了反应和丙二烯产物类型。通过自由基途径开发 1,3-烯炔的不对称 1,4-双官能化将补充以前的方法并支持扩展用于合成不对称丙二烯的工具箱。在此处,我们报告了在铜催化的 1,3-烯炔基 1,4-双官能化的背景下,通过基团转移途径形成的第一个自由基对映选择性丙二烯。该方法解决了不对称自由基化学中一个长期未解决的问题,提供了一种用自由基进行立体控制的重要策略,并为有价值但以前无法获得的手性丙二烯提供了一种新方法。这项工作应该阐明不对称自由基反应,并可能导致其他对映选择性基团转移反应。并为有价值但以前无法获得的手性丙二烯提供了一种新颖的方法。这项工作应该阐明不对称自由基反应,并可能导致其他对映选择性基团转移反应。并为有价值但以前无法获得的手性丙二烯提供了一种新颖的方法。这项工作应该阐明不对称自由基反应,并可能导致其他对映选择性基团转移反应。
更新日期:2020-10-09
中文翻译:

铜催化的 1,3-烯炔的对映选择性自由基 1,4-双官能化
手性丙二烯是重要的结构基序,常见于天然产物、药物和其他有机化合物中。1,3-烯炔的不对称1,4-双官能化是构建轴向手性和从非手性底物产生取代的手性丙二烯的一种很有前景的策略。然而,1,3-烯炔的 1,4-双官能化的先前技术状态集中在丙二烯基阴离子途径上。因此,在第二个功能化步骤中只能将亲电子试剂引入丙二烯主链,从而限制了反应和丙二烯产物类型。通过自由基途径开发 1,3-烯炔的不对称 1,4-双官能化将补充以前的方法并支持扩展用于合成不对称丙二烯的工具箱。在此处,我们报告了在铜催化的 1,3-烯炔基 1,4-双官能化的背景下,通过基团转移途径形成的第一个自由基对映选择性丙二烯。该方法解决了不对称自由基化学中一个长期未解决的问题,提供了一种用自由基进行立体控制的重要策略,并为有价值但以前无法获得的手性丙二烯提供了一种新方法。这项工作应该阐明不对称自由基反应,并可能导致其他对映选择性基团转移反应。并为有价值但以前无法获得的手性丙二烯提供了一种新颖的方法。这项工作应该阐明不对称自由基反应,并可能导致其他对映选择性基团转移反应。并为有价值但以前无法获得的手性丙二烯提供了一种新颖的方法。这项工作应该阐明不对称自由基反应,并可能导致其他对映选择性基团转移反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号