当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurements of Mineral Solubilities in Two Systems MgCl2 + SrCl2 + H2O and NaCl + MgCl2 + SrCl2 + H2O at 288 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-10-09 , DOI: 10.1021/acs.jced.0c00617 Yun-Yun Gao 1 , Xiao-Feng Guo 1 , Han-Zhong Zhang 1 , Shi-Hua Sang 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-10-09 , DOI: 10.1021/acs.jced.0c00617 Yun-Yun Gao 1 , Xiao-Feng Guo 1 , Han-Zhong Zhang 1 , Shi-Hua Sang 1, 2
Affiliation
|
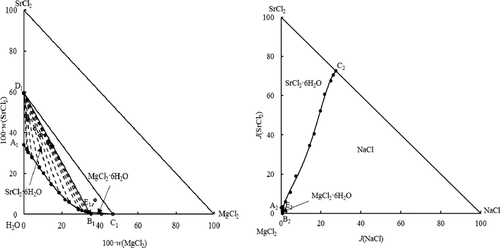
Mineral solubilities in the ternary system MgCl2 + SrCl2 + H2O and the quaternary system NaCl + MgCl2 + SrCl2 + H2O at 288 K were measured using the isothermal dissolution equilibrium method. Two phase diagrams were drawn and both of them were of simple cosaturation type. The ternary system MgCl2 + SrCl2 + H2O contains one invariant point, two isothermal solubility curves, and two crystallization fields (MgCl2·6H2O and SrCl2·6H2O). The quaternary system NaCl + MgCl2 + SrCl2 + H2O consists of one invariant point, three isothermal solubility curves, and three crystallization fields (NaCl, MgCl2·6H2O, and SrCl2·6H2O). At the same time, the comparisons of the ternary system MgCl2 + SrCl2 + H2O at different temperatures were conducted. The results show that the solubility of magnesium chloride changes greatly with the increase of the temperature, which can guide the separation of magnesium chloride and strontium chloride in brines.
中文翻译:

在288 K的MgCl 2 + SrCl 2 + H 2 O和NaCl + MgCl 2 + SrCl 2 + H 2 O两个体系中矿物溶解度的测量
使用等温溶解平衡法测量了三元体系MgCl 2 + SrCl 2 + H 2 O和四元体系NaCl + MgCl 2 + SrCl 2 + H 2 O在288 K下的矿物溶解度。绘制了两个相图,它们都是简单的饱和型。三元体系MgCl 2 + SrCl 2 + H 2 O包含一个不变点,两个等温溶解度曲线和两个结晶场(MgCl 2 ·6H 2 O和SrCl 2 ·6H 2 O)。四元体系NaCl + MgCl 2 + SrCl2 + H 2 O由一个不变点,三个等温溶解度曲线和三个结晶场(NaCl,MgCl 2 ·6H 2 O和SrCl 2 ·6H 2 O)组成。同时进行了三元体系MgCl 2 + SrCl 2 + H 2 O在不同温度下的比较。结果表明,氯化镁的溶解度随温度的升高而变化很大,这可以指导氯化镁和氯化锶在盐水中的分离。
更新日期:2020-11-12
中文翻译:

在288 K的MgCl 2 + SrCl 2 + H 2 O和NaCl + MgCl 2 + SrCl 2 + H 2 O两个体系中矿物溶解度的测量
使用等温溶解平衡法测量了三元体系MgCl 2 + SrCl 2 + H 2 O和四元体系NaCl + MgCl 2 + SrCl 2 + H 2 O在288 K下的矿物溶解度。绘制了两个相图,它们都是简单的饱和型。三元体系MgCl 2 + SrCl 2 + H 2 O包含一个不变点,两个等温溶解度曲线和两个结晶场(MgCl 2 ·6H 2 O和SrCl 2 ·6H 2 O)。四元体系NaCl + MgCl 2 + SrCl2 + H 2 O由一个不变点,三个等温溶解度曲线和三个结晶场(NaCl,MgCl 2 ·6H 2 O和SrCl 2 ·6H 2 O)组成。同时进行了三元体系MgCl 2 + SrCl 2 + H 2 O在不同温度下的比较。结果表明,氯化镁的溶解度随温度的升高而变化很大,这可以指导氯化镁和氯化锶在盐水中的分离。









































 京公网安备 11010802027423号
京公网安备 11010802027423号