当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Osteoblastic Wntless deletion differentially regulates the fate and functions of bone marrow-derived stem cells in relation to age
STEM CELLS ( IF 4.0 ) Pub Date : 2020-10-10 , DOI: 10.1002/stem.3289 Sher Bahadur Poudel 1 , Han-Sol So 2 , Hyun-Jaung Sim 2 , Joon-Seok Cho 3 , Eui-Sic Cho 4 , Young-Mi Jeon 4 , Sung-Ho Kook 2 , Jeong-Chae Lee 2, 4
STEM CELLS ( IF 4.0 ) Pub Date : 2020-10-10 , DOI: 10.1002/stem.3289 Sher Bahadur Poudel 1 , Han-Sol So 2 , Hyun-Jaung Sim 2 , Joon-Seok Cho 3 , Eui-Sic Cho 4 , Young-Mi Jeon 4 , Sung-Ho Kook 2 , Jeong-Chae Lee 2, 4
Affiliation
|
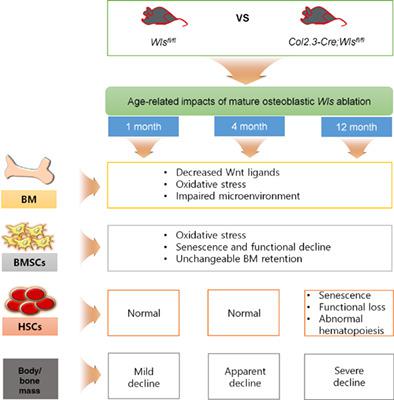
Although functional association between Wnt signaling and bone homeostasis has been well described through genetic ablation of Wntless (Wls), the mechanisms of how osteoblastic Wls regulates the fate of bone marrow stromal cells (BMSCs) and hematopoietic stem cells (HSCs) in relation to age are not yet understood. Here, we generated Col2.3‐Cre;Wlsfl/fl mice that were free from premature lethality and investigated age‐related impacts of osteoblastic Wls deficiency on hematopoiesis, BM microenvironment, and maintenance of BMSCs (also known as BM‐derived mesenchymal stem/stromal cells) and HSCs. Ablation of osteoblastic Wls deteriorated BM microenvironment and bone mass accrual along with age‐independent effects on functions of BMSCs. Osteoblastic Wls deletion impaired HSC repopulation and progeny with skewing toward myeloid lineage cells only at old stage. As proven by hallmarks of stem cell senescence, osteoblastic Wls ablation differentially induced senescence of BMSCs and HSCs in relation to age without alteration in their BM frequency. Our findings support that deletion of Wls in Col2.3‐expressing cells induces senescence of BMSCs and impairs BM microenvironment in age‐independent manner. Overall, long‐term deterioration in BM microenvironment contributes to age‐related HSC senescence with impaired progeny and hematopoiesis, which also suggests possible roles of osteoblastic Wls on the maintenance of BM HSCs.
中文翻译:

成骨细胞 Wntless 缺失差异调节与年龄相关的骨髓源性干细胞的命运和功能
尽管 Wnt 信号转导与骨稳态之间的功能关联已经通过 Wntless (Wls) 的基因消融得到了很好的描述,但成骨细胞 Wls 如何调节与年龄相关的骨髓基质细胞 (BMSC) 和造血干细胞 (HSC) 命运的机制还不明白。在这里,我们生成了 Col2.3-Cre;Wlsfl/fl 小鼠,这些小鼠没有过早致死,并研究了成骨细胞 Wls 缺乏对造血、BM 微环境和 BMSC(也称为 BM 衍生间充质干/基质细胞)和 HSC。成骨细胞 Wls 的消融恶化了 BM 微环境和骨量增加以及对 BMSCs 功能的与年龄无关的影响。成骨细胞 Wls 缺失损害了 HSC 的再增殖和后代,仅在老年阶段向髓系细胞倾斜。正如干细胞衰老的标志所证明的那样,成骨细胞 Wls 消融在不改变其 BM 频率的情况下,随年龄不同诱导 BMSC 和 HSC 衰老。我们的研究结果支持表达 Col2.3 的细胞中 Wls 的缺失诱导 BMSCs 衰老并以与年龄无关的方式损害 BM 微环境。总体而言,BM 微环境的长期恶化导致与年龄相关的 HSC 衰老,后代和造血功能受损,这也表明成骨细胞 Wls 在维持 BM HSC 方面可能发挥作用。成骨细胞 Wls 消融与年龄相关的 BMSC 和 HSC 的衰老不同,而它们的 BM 频率没有改变。我们的研究结果支持表达 Col2.3 的细胞中 Wls 的缺失诱导 BMSCs 衰老并以与年龄无关的方式损害 BM 微环境。总体而言,BM 微环境的长期恶化导致与年龄相关的 HSC 衰老,后代和造血功能受损,这也表明成骨细胞 Wls 在维持 BM HSC 方面可能发挥作用。成骨细胞 Wls 消融与年龄相关的 BMSC 和 HSC 的衰老不同,而它们的 BM 频率没有改变。我们的研究结果支持表达 Col2.3 的细胞中 Wls 的缺失诱导 BMSCs 衰老并以与年龄无关的方式损害 BM 微环境。总体而言,BM 微环境的长期恶化导致与年龄相关的 HSC 衰老,后代和造血功能受损,这也表明成骨细胞 Wls 在维持 BM HSC 方面可能发挥作用。
更新日期:2020-10-10
中文翻译:

成骨细胞 Wntless 缺失差异调节与年龄相关的骨髓源性干细胞的命运和功能
尽管 Wnt 信号转导与骨稳态之间的功能关联已经通过 Wntless (Wls) 的基因消融得到了很好的描述,但成骨细胞 Wls 如何调节与年龄相关的骨髓基质细胞 (BMSC) 和造血干细胞 (HSC) 命运的机制还不明白。在这里,我们生成了 Col2.3-Cre;Wlsfl/fl 小鼠,这些小鼠没有过早致死,并研究了成骨细胞 Wls 缺乏对造血、BM 微环境和 BMSC(也称为 BM 衍生间充质干/基质细胞)和 HSC。成骨细胞 Wls 的消融恶化了 BM 微环境和骨量增加以及对 BMSCs 功能的与年龄无关的影响。成骨细胞 Wls 缺失损害了 HSC 的再增殖和后代,仅在老年阶段向髓系细胞倾斜。正如干细胞衰老的标志所证明的那样,成骨细胞 Wls 消融在不改变其 BM 频率的情况下,随年龄不同诱导 BMSC 和 HSC 衰老。我们的研究结果支持表达 Col2.3 的细胞中 Wls 的缺失诱导 BMSCs 衰老并以与年龄无关的方式损害 BM 微环境。总体而言,BM 微环境的长期恶化导致与年龄相关的 HSC 衰老,后代和造血功能受损,这也表明成骨细胞 Wls 在维持 BM HSC 方面可能发挥作用。成骨细胞 Wls 消融与年龄相关的 BMSC 和 HSC 的衰老不同,而它们的 BM 频率没有改变。我们的研究结果支持表达 Col2.3 的细胞中 Wls 的缺失诱导 BMSCs 衰老并以与年龄无关的方式损害 BM 微环境。总体而言,BM 微环境的长期恶化导致与年龄相关的 HSC 衰老,后代和造血功能受损,这也表明成骨细胞 Wls 在维持 BM HSC 方面可能发挥作用。成骨细胞 Wls 消融与年龄相关的 BMSC 和 HSC 的衰老不同,而它们的 BM 频率没有改变。我们的研究结果支持表达 Col2.3 的细胞中 Wls 的缺失诱导 BMSCs 衰老并以与年龄无关的方式损害 BM 微环境。总体而言,BM 微环境的长期恶化导致与年龄相关的 HSC 衰老,后代和造血功能受损,这也表明成骨细胞 Wls 在维持 BM HSC 方面可能发挥作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号