当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of some 1,3‐benzoxazol‐2(3H)‐one hybrid molecules as potential antioxidant and urease inhibitors
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-10-08 , DOI: 10.1002/jhet.4165 Fatih Yilmaz 1 , Emre Menteşe 2 , Bahar Bilgin Sökmen 3
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-10-08 , DOI: 10.1002/jhet.4165 Fatih Yilmaz 1 , Emre Menteşe 2 , Bahar Bilgin Sökmen 3
Affiliation
|
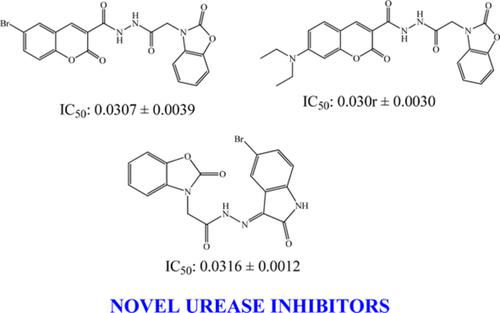
A new series of 1,3‐benzoxazol‐2(3H)‐one hybrid compounds, including coumarin, isatin 1,3,4‐triazole and 1,3,4‐thiadiazole moieties, were synthesized and biologically evaluated for their antioxidant capacities and anti‐urease properties. The synthesized benzoxazole‐coumarin (6a–e) and benzoxazole‐isatin (10a–c) hybrids showed remarkable urease inhibitory activities with IC50 (μM), ranging from 0.0306 ± 0.0030 to 0.0402 ± 0.0030, while IC50 of standard thiourea is 0.5027 ± 0.0293. The synthesized benzoxazole‐triazole (8a–c) and benzoxazole‐thiadiazole (9a–c) hybrids showed similar urease inhibitory activities with IC50 (μM), ranging from 0.3861 ± 0.0379 to 0.5126 ± 0.0345. The antioxidant activity of the synthesized compounds was evaluated for their antioxidant activities, such as reducing power and ABTS (2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) diammonium salt) radical scavenging. The results of ABTS radical scavenging activities of some of the synthesized molecules showed higher activities than standard Trolox, SC50 (μM) = 213.04 ± 18.12. One benzoxazole‐coumarin (6f), two benzoxazole‐isothiocyanate (7b, 7c), and two benzoxazole‐triazole (8b, 8c) derivatives showed higher activities (SC50 (μM) values, 82.07 ± 10.34, 120.19 ± 7.30, 104.58 ± 10.55, 153.26 ± 7.14, and 144.82 ± 10.68, respectively) than standard Trolox, (SC50 (μM) = 213.04 ± 18.12).
中文翻译:

某些1,3-苯并恶唑-2(3H)-1杂合分子的合成和生物学评估作为潜在的抗氧化剂和脲酶抑制剂
合成了一系列新的1,3-苯并恶唑-2(3H)-杂合化合物,包括香豆素,靛红1,3,4-三唑和1,3,4-噻二唑部分,并对其生物学性能进行了评估。抗脲酶特性。合成的苯并恶唑香豆素(6a–e)和苯并恶唑·isatin(10a–c)杂种表现出显着的脲酶抑制活性,IC 50(μM)为0.0306±0.0030至0.0402±0.0030,而标准硫脲的IC 50为0.5027 ±0.0293。合成的苯并恶唑-三唑(8a–c)和苯并恶唑–噻二唑(9a–c)杂种显示出对尿素酶的抑制活性与IC 50相似(μM),范围从0.3861±0.0379到0.5126±0.0345。评估了合成化合物的抗氧化活性,如还原力和ABTS(2,2'-叠氮基-双(3-乙基苯并噻唑啉-6-磺酸)二铵盐)清除自由基的活性。一些合成分子的ABTS自由基清除活性结果显示出比标准Trolox更高的活性,SC 50(μM)= 213.04±18.12。一种苯并恶唑香豆素(6f),两种苯并恶唑异硫氰酸酯(7b,7c)和两种苯并恶唑三唑(8b,8c)衍生物显示出更高的活性(SC 50(μM)值分别比标准Trolox(SC 50(μM)= 213.04±18.12)分别为82.07±10.34、120.19±7.30、104.58±10.55、153.26±7.14和144.82±10.68 。
更新日期:2020-10-08
中文翻译:

某些1,3-苯并恶唑-2(3H)-1杂合分子的合成和生物学评估作为潜在的抗氧化剂和脲酶抑制剂
合成了一系列新的1,3-苯并恶唑-2(3H)-杂合化合物,包括香豆素,靛红1,3,4-三唑和1,3,4-噻二唑部分,并对其生物学性能进行了评估。抗脲酶特性。合成的苯并恶唑香豆素(6a–e)和苯并恶唑·isatin(10a–c)杂种表现出显着的脲酶抑制活性,IC 50(μM)为0.0306±0.0030至0.0402±0.0030,而标准硫脲的IC 50为0.5027 ±0.0293。合成的苯并恶唑-三唑(8a–c)和苯并恶唑–噻二唑(9a–c)杂种显示出对尿素酶的抑制活性与IC 50相似(μM),范围从0.3861±0.0379到0.5126±0.0345。评估了合成化合物的抗氧化活性,如还原力和ABTS(2,2'-叠氮基-双(3-乙基苯并噻唑啉-6-磺酸)二铵盐)清除自由基的活性。一些合成分子的ABTS自由基清除活性结果显示出比标准Trolox更高的活性,SC 50(μM)= 213.04±18.12。一种苯并恶唑香豆素(6f),两种苯并恶唑异硫氰酸酯(7b,7c)和两种苯并恶唑三唑(8b,8c)衍生物显示出更高的活性(SC 50(μM)值分别比标准Trolox(SC 50(μM)= 213.04±18.12)分别为82.07±10.34、120.19±7.30、104.58±10.55、153.26±7.14和144.82±10.68 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号