当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TRIM4 interacts with TRPM8 and regulates its channel function through K423‐mediated ubiquitination
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-10-09 , DOI: 10.1002/jcp.30065 Yuan Huang 1 , Shunyao Li 1 , Zhenhua Jia 1 , Shi Li 1 , Wenzao He 1 , Cefan Zhou 1 , Rui Zhang 1 , Rui Xu 1 , Bo Sun 1 , Declan William Ali 2 , Marek Michalak 3 , Xing-Zhen Chen 4 , Jingfeng Tang 1
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-10-09 , DOI: 10.1002/jcp.30065 Yuan Huang 1 , Shunyao Li 1 , Zhenhua Jia 1 , Shi Li 1 , Wenzao He 1 , Cefan Zhou 1 , Rui Zhang 1 , Rui Xu 1 , Bo Sun 1 , Declan William Ali 2 , Marek Michalak 3 , Xing-Zhen Chen 4 , Jingfeng Tang 1
Affiliation
|
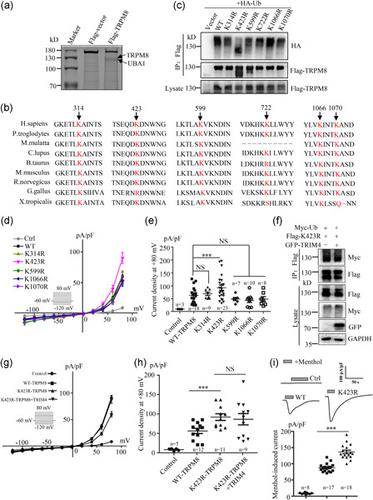
Transient receptor potential melastatin member 8 (TRPM8), a Ca2+‐permeable nonselective cation channel activated by cold and cooling agents, mediates allodynia. Dysfunction or abnormal expression of TRPM8 has been found in several human cancers. The role of ubiquitination in the regulation of TRPM8 function remains poorly understood. Here, we identified the ubiquitin (Ub)‐ligase E3, tripartite motif‐containing 4 (TRIM4), as a novel interaction partner of TRPM8 and confirmed that the TRIM4–TRPM8 interaction was mediated through the SPRY domain of TRIM4. Patch‐clamp assays showed that TRIM4 negatively regulates TRPM8‐mediated currents in HEK293 cells. Moreover, TRIM4 reduced the expression of TRPM8 on the cell surface by promoting the K63‐linked ubiquitination of TRPM8. Further analyses revealed that the TRPM8 N‐terminal lysine residue at 423 was the major ubiquitination site that mediates its functional regulation by TRIM4. A Ub‐activating enzyme E1, Ub‐like modifier‐activating enzyme 1 (UBA1), was also found to interact with TRPM8, thereby regulating its channel function and ubiquitination. In addition, knockdown of UBA1 impaired the regulation of TRPM8 ubiquitination and function by TRIM4. Thus, this study demonstrates that TRIM4 downregulates TRPM8 via K423‐mediated TRPM8 ubiquitination and requires UBA1 to regulate TRPM8.
中文翻译:

TRIM4 与 TRPM8 相互作用并通过 K423 介导的泛素化调节其通道功能
瞬时受体电位褪黑素成员 8 (TRPM8),一种 Ca 2+-由冷剂和凉味剂激活的可渗透的非选择性阳离子通道,介导异常性疼痛。已经在几种人类癌症中发现了 TRPM8 的功能障碍或异常表达。泛素化在 TRPM8 功能调节中的作用仍然知之甚少。在这里,我们确定了泛素 (Ub)-连接酶 E3,包含三联基序的 4 (TRIM4),作为 TRPM8 的新型相互作用伙伴,并证实 TRIM4-TRPM8 相互作用是通过 TRIM4 的 SPRY 结构域介导的。膜片钳试验表明,TRIM4 在 HEK293 细胞中负调节 TRPM8 介导的电流。此外,TRIM4 通过促进 K63 相关的 TRPM8 泛素化来降低细胞表面 TRPM8 的表达。进一步的分析表明,TRPM8 N 端 423 位的赖氨酸残基是主要的泛素化位点,通过 TRIM4 介导其功能调节。还发现 Ub 激活酶 E1,Ub 样修饰激活酶 1(UBA1)与 TRPM8 相互作用,从而调节其通道功能和泛素化。此外,UBA1 的敲低损害了 TRPM8 泛素化和 TRIM4 功能的调节。因此,这项研究表明,TRIM4 通过 K423 介导的 TRPM8 泛素化下调 TRPM8,并需要 UBA1 来调节 TRPM8。
更新日期:2020-10-09
中文翻译:

TRIM4 与 TRPM8 相互作用并通过 K423 介导的泛素化调节其通道功能
瞬时受体电位褪黑素成员 8 (TRPM8),一种 Ca 2+-由冷剂和凉味剂激活的可渗透的非选择性阳离子通道,介导异常性疼痛。已经在几种人类癌症中发现了 TRPM8 的功能障碍或异常表达。泛素化在 TRPM8 功能调节中的作用仍然知之甚少。在这里,我们确定了泛素 (Ub)-连接酶 E3,包含三联基序的 4 (TRIM4),作为 TRPM8 的新型相互作用伙伴,并证实 TRIM4-TRPM8 相互作用是通过 TRIM4 的 SPRY 结构域介导的。膜片钳试验表明,TRIM4 在 HEK293 细胞中负调节 TRPM8 介导的电流。此外,TRIM4 通过促进 K63 相关的 TRPM8 泛素化来降低细胞表面 TRPM8 的表达。进一步的分析表明,TRPM8 N 端 423 位的赖氨酸残基是主要的泛素化位点,通过 TRIM4 介导其功能调节。还发现 Ub 激活酶 E1,Ub 样修饰激活酶 1(UBA1)与 TRPM8 相互作用,从而调节其通道功能和泛素化。此外,UBA1 的敲低损害了 TRPM8 泛素化和 TRIM4 功能的调节。因此,这项研究表明,TRIM4 通过 K423 介导的 TRPM8 泛素化下调 TRPM8,并需要 UBA1 来调节 TRPM8。











































 京公网安备 11010802027423号
京公网安备 11010802027423号