当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and activity against Staphylococcus epidermidis of 5‐chloro‐2‐ or 5‐chloro‐4‐methyl‐9H‐xanthen‐9‐one and some of its derivatives
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-10-08 , DOI: 10.1111/cbdd.13803 Gabriela Mazur 1 , Iwona Skiba-Kurek 2 , Elżbieta Karczewska 2 , Katarzyna Pańczyk-Straszak 1 , Joanna Jaworska 1 , Anna M Waszkielewicz 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-10-08 , DOI: 10.1111/cbdd.13803 Gabriela Mazur 1 , Iwona Skiba-Kurek 2 , Elżbieta Karczewska 2 , Katarzyna Pańczyk-Straszak 1 , Joanna Jaworska 1 , Anna M Waszkielewicz 1
Affiliation
|
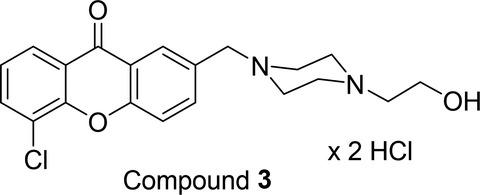
Ten new xanthone derivatives have been designed and synthesized for their potential antibacterial activity. All compounds have been screened against Staphylococcus epidermidis strains ATCC 12228 and clinical K/12/8915. The highest antibacterial activity was observed for compound 3: 5‐chloro‐2‐((4‐(2‐hydroxyethyl)piperazin‐1‐yl)methyl)‐9H‐xanthen‐9‐one dihydrochloride, exhibiting MIC of 0.8 µg/ml against ATCC 12228 strain, compared to linezolid (0.8 µg/ml), ciprofloxacin (0.2 µg/ml) or trimethoprim and sulfamethoxazole (0.8 µg/ml). For the most active compound 3, genotoxicity assay with use of Salmonella enterica serovar Typhimurium revealed safety in terms of genotoxicity at concentration 75 µg/ml and antibacterial activity against Salmonella at all higher concentrations. A final in silico prediction of skin metabolism of compound 3 seems promising, indicating stability of the xanthone moiety in the metabolism process.
中文翻译:

5-chloro-2- 或 5-chloro-4-methyl-9H-xanthen-9-one 及其一些衍生物的设计、合成和抗表皮葡萄球菌的活性
十种新的氧杂蒽酮衍生物因其潜在的抗菌活性而被设计和合成。所有化合物均已针对表皮葡萄球菌菌株 ATCC 12228 和临床 K/12/8915进行筛选。化合物3 的抗菌活性最高:5-氯-2-((4-(2-羟乙基)哌嗪-1-基)甲基)-9 H -xanthen-9-one 二盐酸盐,MIC 为 0.8 µg/与利奈唑胺 (0.8 µg/ml)、环丙沙星 (0.2 µg/ml) 或甲氧苄氨嘧啶和磺胺甲恶唑 (0.8 µg/ml) 相比。对于活性最强的化合物3,使用鼠伤寒沙门氏菌血清型进行遗传毒性试验显示在 75 µg/ml 浓度下的遗传毒性和所有更高浓度下对沙门氏菌的抗菌活性方面的安全性。化合物3皮肤代谢的最终计算机预测似乎很有希望,表明氧杂蒽酮部分在代谢过程中的稳定性。
更新日期:2020-10-08
中文翻译:

5-chloro-2- 或 5-chloro-4-methyl-9H-xanthen-9-one 及其一些衍生物的设计、合成和抗表皮葡萄球菌的活性
十种新的氧杂蒽酮衍生物因其潜在的抗菌活性而被设计和合成。所有化合物均已针对表皮葡萄球菌菌株 ATCC 12228 和临床 K/12/8915进行筛选。化合物3 的抗菌活性最高:5-氯-2-((4-(2-羟乙基)哌嗪-1-基)甲基)-9 H -xanthen-9-one 二盐酸盐,MIC 为 0.8 µg/与利奈唑胺 (0.8 µg/ml)、环丙沙星 (0.2 µg/ml) 或甲氧苄氨嘧啶和磺胺甲恶唑 (0.8 µg/ml) 相比。对于活性最强的化合物3,使用鼠伤寒沙门氏菌血清型进行遗传毒性试验显示在 75 µg/ml 浓度下的遗传毒性和所有更高浓度下对沙门氏菌的抗菌活性方面的安全性。化合物3皮肤代谢的最终计算机预测似乎很有希望,表明氧杂蒽酮部分在代谢过程中的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号