Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-09 , DOI: 10.1016/j.tet.2020.131660 Qi Gu , Luyao Kong , Lin Yang , Lili Zhu , Ran Hong
|
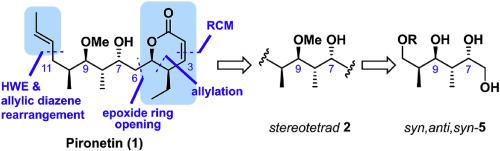
Pironetin is a unique α-tubulin inhibitor and has been of utmost interest in the synthetic community. Based on the enabling method to access various stereotriads and stereotetrads, we devised a new total synthesis of pironetin, a total 13 steps from commercially available (S)-Roche ester, representing one of the shortest synthetic routes reported to date. The facile fragmentation induced by the deprotonation of the pyrone ring at C4 led us to reorient the installation of the remote enone moiety prior to the lactone ring formation. Moreover, the undesired Michael addition of a phosphate reagent to the acrylate guided us to introduce a sterically demanding cinnamate motif that was also found to facilitate the selective removal of the TBDPS group. The evolution of the synthetic route underlines that the experimental execution of the reactivity of various functional groups is instrumental to devise a successful synthesis.
中文翻译:

以立体四心为中心的吡咯丁酮方法:死胡同,绕道而行以及合成策略的演变
Pironetin是一种独特的α-微管蛋白抑制剂,在合成界引起了极大关注。基于访问各种立体三联体和立体四联体的使能方法,我们设计了一种新的吡咯丁酮全合成,从市售可得共13步(S)-罗氏酯,代表迄今为止报道的最短的合成路线之一。C4处的吡喃环去质子化诱导的易碎片段使我们在形成内酯环之前重新定位了远端烯酮部分的安装方向。此外,不希望的迈克尔酯向丙烯酸酯的磷酸酯加成反应指导我们引入了空间上要求的肉桂酸酯基序,该基序也被发现有助于选择性除去TBDPS基团。合成路线的演变突显了各种官能团反应性的实验执行对于设计成功的合成至关重要。










































 京公网安备 11010802027423号
京公网安备 11010802027423号