Molecular Catalysis ( IF 4.6 ) Pub Date : 2020-10-09 , DOI: 10.1016/j.mcat.2020.111237 Yanwei Cao , Chaoren Shen , Jie Min , Lin He , Xinxin Tian
|
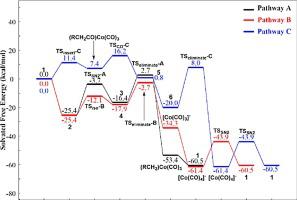
The mechanism of cobalt-catalyzed methoxycarbonylation of bromoacetonitrile and methyl 2-halogen acetate was computationally investigated by comparing three different reaction pathways with the consideration of solvation and countercation effect. Pathway B, which goes through the addition of MeO− to (RCH2)Co(CO)4 and the elimination of [(RCH2)Co(CO)3(COOMe)]− to afford ester, is found to be the most kinetically favored. The previously proposed pathway A, which goes through twice SN2 reaction, and herein proposed pathway C, which undergoes the conventional migratory CO insertion of (RCH2)Co(CO)4 and further methanolysis of (RCH2CO)Co(CO)4 intermediate, are less favored due to their higher barrier. The thermodynamic contribution of countercation is distinct, but the mechanism preference still keeps.
中文翻译:

钴卤代烷氧羰基化反应机理的计算研究
通过比较三种不同的反应途径并考虑了溶剂化和反阳离子作用,通过计算研究了钴催化的溴乙腈和乙酸2-卤代甲基的甲氧基羰基化的机理。途径乙,其穿过另外的MeO的-至(RCH 2)的Co(CO)4和[(RCH消除2)的Co(CO)3(COOMe)] - ,得到酯,被发现是最在动力学上受到青睐。先前提出的途径A(经历两次S N 2反应)和本文提出的途径C由于其较高的阻隔性,其经历了(RCH 2)Co(CO)4的常规迁移CO插入和(RCH 2 CO)Co(CO)4中间体的进一步甲醇分解,因此较不受欢迎。抗衡阳离子的热力学贡献是独特的,但机理仍然保持不变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号