Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-10-09 , DOI: 10.1016/j.jsb.2020.107645 Khaja Faisal Tarique 1 , Suneeta Devi 2 , Priya Tomar 2 , Mohammad Farhan Ali 2 , Syed Arif Abdul Rehman 3 , Samudrala Gourinath 2
|
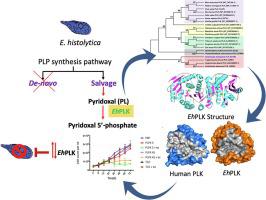
Pyridoxal 5′-phosphate (PLP) is the active form of vitamin B6 and a cofactor for more than 140 enzymes. This coenzyme plays a pivotal role in catalysis of various enzymatic reactions that are critical for the survival of organisms. Entamoeba histolytica depends on the uptake of pyridoxal (PL), a B6 vitamer from the external environment which is then phosphorylated by pyridoxal kinase (EhPLK) to form PLP via the salvage pathway. E. histolytica cannot synthesise vitamin B6 de-novo, and also lacks pyridoxine 5′-phosphate oxidase, a salvage pathway enzyme required to produce PLP from pyridoxine phosphate (PNP) and pyridoxamine phosphate (PMP). Analysing the importance of PLK in E. histolytica, we have determined the high-resolution crystal structures of the dimeric pyridoxal kinase in apo, ADP-bound, and PLP-bound states. These structures provided a snapshot of the transition state and help in understanding the reaction mechanism in greater detail. The EhPLK structure significantly differed from the human homologue at its PLP binding site, and the phylogenetic study also revealed its divergence from human PLK. Further, gene regulation of EhPLK using sense and antisense RNA showed that any change in optimal level is harmful to the pathogen. Biochemical and in vivo studies unveiled EhPLK to be essential for this pathogen, while the molecular differences with human PLK structure can be exploited for the structure-guided design of EhPLK inhibitors.
中文翻译:

对溶组织内阿米巴吡哆醛激酶的表征和功能见解,一种对其生存必不可少的酶
5'-磷酸吡哆醛 (PLP) 是维生素 B 6的活性形式,是140 多种酶的辅助因子。这种辅酶在催化各种对生物体生存至关重要的酶促反应中起着关键作用。溶组织内阿米巴依赖于吡哆醛 (PL),一种来自外部环境的 B 6维生素,然后被吡哆醛激酶 (EhPLK) 磷酸化,通过补救途径形成 PLP。E. histolytica不能从头合成维生素 B 6 ,也缺乏吡哆醇 5'-磷酸氧化酶,这是一种从磷酸吡哆醇 (PNP) 和磷酸吡哆胺 (PMP) 产生 PLP 所需的补救途径酶。分析 PLK 的重要性E. histolytica,我们已经确定了在 apo、ADP 结合和 PLP 结合状态下二聚吡哆醛激酶的高分辨率晶体结构。这些结构提供了过渡态的快照,有助于更详细地了解反应机制。EhPLK 结构在其 PLP 结合位点与人类同源物显着不同,系统发育研究也揭示了它与人类 PLK 的差异。此外,使用有义和反义 RNA 对 EhPLK 的基因调控表明,最佳水平的任何变化都对病原体有害。生化和体内研究表明 EhPLK 对这种病原体至关重要,而人类 PLK 结构的分子差异可用于 EhPLK 抑制剂的结构引导设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号